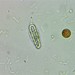
Fotos / Sonidos
Observ.
davidfbirdDescripción
On the bark of a linden tree, along with patches of Graphis scripta. Photobiont is Trentepohlia - this very humid valley is rich with pure patches of Trentepohlia. The small piece of loose bark I found had no apothecia, but did have two pycnidia. Conidia were hyaline, bacilliform, about 4-5 microns long and 1.2 microns wide.
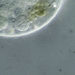
Fotos / Sonidos
Observ.
dgborinDescripción
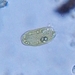
Sample taken on 2024-07-20.
Slowing solution 0.5% Methyl cellulose
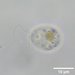
Fotos / Sonidos
Qué
Género FrontoniaObserv.
crseaquistDescripción
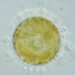
Water sample (freshwater) was taken on 07/20/2024 using a turkey baster..
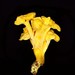
Fotos / Sonidos
Qué
Matachicharras del Este (Sphecius speciosus)Observ.
mnold1Descripción
Last image shows the Eastern Cicada-killer on the pile of dirt and gravel excavated while digging its nest. (impressive pile!)
Fotos / Sonidos
Observ.
davidfbirdDescripción
From a macrophyte bed on the lakeshore of a eutrophic lake. No sheath, aerotopes or heterocytes. Motile, 5-5.5 x 8.5 µm W x L. Cells constricted in the middle and at crosswalls. Terminal cells slightly swollen, rounded.
Fotos / Sonidos
Qué
Vida (Vida)Observ.
goldfjnchDescripción
40x objective
sample from fresh water pond
image 4 is sped up 400%
image 5 is sped up 5000%
Fotos / Sonidos
Qué
Spirulina majorObserv.
davidfbirdDescripción
From a stagnant stormwater pond. Coil length 2.5-3.2 µ, coil diameter 3.6-4.1, cell width 1.8 µ, filaments isolated, actively motile. The gif is real-time.
Fotos / Sonidos
Qué
Copépodos (Subclase Copepoda)Observ.
pinefrogDescripción
Collected from a permanent freshwater woodland pond.
Fotos / Sonidos
Qué
Mapache (Procyon lotor)Observ.
mnold1Descripción
4 young raccoons (siblings?) foraging together in a relatively tight group - fun to watch!
Fotos / Sonidos
Qué
Diatomeas (Clase Bacillariophyceae)Observ.
kenzieb87Descripción
10-40x Objective
Freshwater
Dyes: Bismarck Brown, Neutral Red
AmScope B120C Microscope
MD100 AmScope Camera
I am still new to microscopy, staining, and identifying microscopic organisms in general. Any tips for bettering my observations would be greatly appreciated. I also appreciate when people explain their identifications.
Fotos / Sonidos
Observ.
davidfbirdDescripción
From a surface sample collected on the shoreline, where there was an accumulation of pollen. Ovoid but very flexible ciliates with a figure-of-6 spiral opening to the mouth, watchglass-shaped lens over the X-shaped pigment spot, large lateral vacuole and baguette-shaped nucleus. These observations are of a phase where they tend to rotate in place. (I'll post separately an entry for the frenetic run-about-madly phase that I just recognized to be this same species. Link: https://inaturalist.ca/observations/223984696). They often develop an indentation at the mid-section, with sometimes an odd little projection. The still shots are screenshots from video footage, to show some of the morphological contortions they exhibit.
Fotos / Sonidos
Observ.
someplantDescripción
Magnification of photos: 600×, 600×, 600×, 600×, 600×
Habitat: Myriophyllum and Spirogyra growing at the bottom of a reservoir.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 600× is 100 µm.
https://www.celestron.com/blogs/knowledgebase/what-is-my-digital-microscopes-field-of-view
The cell body "wobbles" by rocking from side to side while moving. I could see one flagellum, which was trailing.
Fotos / Sonidos
Qué
Vida (Vida)Observ.
crseaquistDescripción
Water sample (freshwater) was taken on 07/10/2022 using a coffee filter to enrich for microorganisms.
Fotos / Sonidos
Observ.
bennett-sDescripción
Cow horn shaped diatom-like organism with longitudinal striations from end-to-end.
The site is a sphagnum bog that abuts a pond.
Fotos / Sonidos
Observ.
mnold1Descripción
The 1st and 2nd photos are enlargements of sections of the colony when first encountered, i.e, when the cells were only loosely associated. Photos 3 through 10 capture the relatively rapid aggregation of the cells into a tight cluster; aggregation appeared near maximum within 1.5 minutes after the start of the observation. I suspect exposure to the focused illumination from the scope somehow triggered the aggregation.
Amazing to witness! Interesting (and fast!) signaling biochemistry at play here! ...or is this similar to the coordinated flight of bird flocks, relying on "visual" cues?
Water sample collected from a vernal stream, near moss-covered rocks and leaf-covered bottom; water depth 4-5 inches with very little flow. Air temp 55F
Observ.
gpmatthewsDescripción
Gastronauta membranaceus
Microscope: Leitz Dialux
Ocular: 10x GF Periplan
Objective: 40/0.70 NPL Fluotar ICT (DIC)
Sample from garden pond
Flash
Qué
Diatomeas (Clase Bacillariophyceae)Observ.
mnold1Descripción
Mag. 400x
Fragment of a tube-dwelling Navicula sp. colony? (Looks more like Navicula than Cymbella and Frustula, both of which have tube-dwelling varieties). I count 7 cells in this package... 8 would make more sense if the number is linked to binary replication. Also interesting is the size variation; e.g. in the 3rd panel from the left one sees a small cell in the foreground with a mix of much larger cells behind (makes one wonder about the replicational relationship among the cells... perhaps there is none).
- On 3/29/2022 a water and moss sample were taken from the submerged section of a boulder in the Billings-Avery Brook. Air temp. 38F
Fotos / Sonidos
Qué
Cianobacterias Espirulina (Género Spirulina)Observ.
maricel-patinoDescripción
Found in water collected from a rice canal.
Qué
Diatomeas (Clase Bacillariophyceae)Observ.
laneallenDescripción
All specimens from the Colorado Front Range.
Scale Bar = 10 µm.
Fotos / Sonidos
Qué
Hongos de Saco, Levaduras Y Parientes (Filo Ascomycota)Observ.
maricel-patinoDescripción
On an orchid leaf.
Fotos / Sonidos
Qué
Euglenas (Filo Euglenozoa)Observ.
valveDescripción
freshwater constructed wetland.
Fotos / Sonidos
Qué
Género DinobryonObserv.
amayakanDescripción
Video: https://youtu.be/sfbkRjOaZI0
Photos are snapshots of the same individual in the video
Cell size: 48 µm in length (excluding flagella)
Site of sample collection: Pavilion, Katsurashima Ryokuchi north pond (a freshwater habitat), Sendai, Japan
Date of sample collection: March 26th 2022
Weather: Cloudy
Water temp.: 9.0°C
pH 6.4
Date of observation: March 27th 2022 (the collected sample in a plastic container was left near a window out of direct sunlight at room temperature until observation)
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Fotos / Sonidos
Qué
Filo ChoanozoaObserv.
mnold1Descripción
Mag. 400x
Choanoflagellates (on a filament of Eunotia sp.).... perhaps genus Salpingoeca as seen here https://www.inaturalist.org/observations/85163828 and http://cfb.unh.edu/phycokey/Choices/Amoebae_Flagellates_Ciliates/Choanoflagellates/SALPINGOECA/Salpingoeca_Image_page.html#pic02.
- A pond-side water sample was taken on 12/27/2021 using a 10µ dip net. About 1/4" of ice had to be broken and pushed aside to access the water. Air temp. 36°F.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
Helical cyanobacteria with crosswalls that are difficult to see, but present (indicated by black arrows where they are distinct). Specie: possibly A. jenneri as seen here https://www.researchgate.net/publication/330579181_Detailed_characterization_of_the_Arthrospira_type_species_separating_commercially_grown_taxa_into_the_new_genus_Limnospira_Cyanobacteria.
A pond edge water sample (freshwater) was taken on 10/1/2021 using a 10 micron dip net to enrich for microorganisms.
Fotos / Sonidos
Observ.
amayakanDescripción
Video: https://youtu.be/gAYpJMw-Zp8
Photos are snapshots of the same individual in the video
Cell size: 16 µm in length, 11 µm in width
Site of sample collection: Pavilion, Katsurashima Ryokuchi north pond (a freshwater habitat), Sendai, Japan
Date of sample collection: March 26th 2022
Weather: Cloudy
Water temp.: 9.0°C
pH 6.4
Date of observation: March 27th 2022 (the collected sample in a plastic container was left near a window out of direct sunlight at room temperature until observation)
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Observ.
plingfactoryDescripción
Bdelloid rotifer with a "saddle" out of detritus; several specimens found, always with a "saddle". Seems not yet described in the literature. More on this here:
https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Bdelloid_20.html
Fotos / Sonidos
Qué
Protozoarios (Reino Protozoa)Observ.
woodilljDescripción
- from marine sample (sand and water), collected at shoreline
- objective(s) (x 10): 600x
- approximate dimensions: body: 15 µm, longest axis; flagella: 23 µm
- video on Vimeo
- ID: ELPB-LGT40
- Body apparently rigid and perhaps bilaterally symmetrical.
- Eight ~~nine~~ (?) flagella emerge from spiky/knobby protuberances.
- Organism is transparent and internal structures are not readily apparent in brightfield.
- 'Walks' or glides along using flagella, sometimes pushing off and 'coasting' (see animated GIF #2 or above-linked video).
- Often, flagella not immediately applied to locomotion are waved busily about (see animated GIF #1 or above-linked video).
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x (progression of focal planes)
Pollen? Anyone one know of a handy online reference for identifying the pollen of various plants?
A pond-edge water sample was collected on 6/2/2021 using a 10 micron dip net to enrich for microorganisms. Air temp. 67F
Fotos / Sonidos
Qué
Colpoda cucullusObserv.
peptolabDescripción
Colpoda cucullus O.F. Muller, 1773 from algae-infused water wrung from fallen leaves that spent the winter submerged on my swimming pool cover. Imaged in Nomarski DIC on Olympus BH2S using SPLANAPO 40 0.95 objective and SPLAN 100 1.25 oil objective with slide oiled to the condenser. The cells measure from 80-100 um in length. We can clearly see the two most important characters which differentiate C. cuculus from other species in the genus: the stellate endosome of the spherical macronucleus and the anterior keel with 9-10 indentations.
" 40-110 um long; anterior keel with 8 to10 indentations; 29-34 ciliary grooves; cilia mostly paired; macro nucleus with a stellate endosome; trichocysts rod-form; usually with abundant food vacuoles; in fresh water with decaying plants" (1).
"Body distinctly reniform in shape, dorso-ventrally flattened. Right body edge strongly convex, left body edge concave often appearing as through a bite had been taken from it. A shallow diagonal somatic groove (not easily visible) originating on the dorsal surface travels round left side to entrance of vestibulum on the flattened ventral surface. Ciliation uniform in longitudinal or oblique orientated grooves. Several notches which denote ciliary grooves often visible on preoral part of left body edge. Caudal cilia may be present on some species. There is a horse-shoe shaped arc of closely-set cilia on the right of the vestibular entrance. Single rounded macronucleus with 1, 2 or 3 micronuclei. Single terminal contractile vacuole. Division takes place in thin-walled cysts, thick-walled protective cysts also formed" (2).
"Morphology Length 40-120 um, average about 80 um; broadly reniform, anterior keel with 8-10 indentations; uniform ciliation; buccal cavity with a deep oral funnel starting at a groove near the left side of the body, the buccal cavity leads to a diagonal groove on the dorsal surface (not evident in the figure), buccal cavity ciliated but without membranes or membranelles; 1 spherical macronucleus, exhibiting a stellate endosome; 1 micronucleus; a single terminal contractile vacuole; in the presence of a good food supply, the body is packed with food inclusions and appears very dark; fission only takes place within reproductive cysts" (3).
" Colpoda cucullus, previously considered a "soil" protozoan, is predominantly a vegetation-associated species that is especially adapted, through its ability to encyst and excyst rapidly, to exploit the fluctuating moisture content of the terrestrial environment. Three means of distribution were discovered which explain the ubiquity of this species. Herbivores consume Colpoda cysts while feeding on infested vegetation and deposit them with their feces; the cysts are transported by honey-bees along with pollen grains; and they are also carried through air like pollen. Dew induces excystment and contains sufficient nutritional substances to support profuse growth and reproduction" (4).
- PROTOZOOLOGY By RICHARD R. KUDO. CHARLES C THOMAS • PUBLISHER Springfield, Illinois. 1954. pp 745-6.
- Colin R. Curds "British and other freshwater ciliated protozoa Part I Ciliophora: Kinetofragminophora" Cambridge University Press, 1982
- CILIATED PROTOZOA. An illustrated guide to the species used as biological indicators in freshwater biology HARTMUT BICK. WORLD HEALTH ORGANIZATION GENEVA 1972 pp 64-5.
- Colpoda cucullus: A Terrestrial Aquatic.Jo Anne Mueller, Wayne P. Mueller. The American Midland N
Fotos / Sonidos
Qué
Hongos (Reino Fungi)Observ.
ikhomDescripción
Black mold (anamorphic fungi) on very wet wood.
Conidia dark, measure in H2O
(5.6) 5.7 - 7.3 (7.5) × (3.1) 3.15 - 3.7 (4) µm
Q = (1.4) 1.6 - 2.2 (2.3) ; N = 20
Me = 6.5 × 3.5 µm ; Qe = 1.9
Fotos / Sonidos
Qué
Algas Doradas (Clase Chrysophyceae)Observ.
zookanthosDescripción
Possibly the same thing as:
https://www.inaturalist.org/observations/36287678
https://www.inaturalist.org/observations/36348371
Fotos / Sonidos
Observ.
heelsplitterDescripción
Growing on and apparently deforming a Cantharellus minor fruiting body. Conidiophores difficult to view. Conidia hyaline, thick-walled, warty and with 2 guttules. Conidia measurements: (17.8) 19.8 – 22.4 (23.6) × (9.5) 11.1 – 12.4 (12.7) µm, Q = (1.6) 1.64 – 1.9 (2); N = 30, Me = 21 × 11.7 µm; Qe = 1.8
Fotos / Sonidos
Qué
Cianobacteria Nostoc (Nostoc commune)Observ.
davidfbirdDescripción
Large patch on flooded floor of the abandoned limestone quarry. Lots of heterocytes.
Fotos / Sonidos
Qué
Escarabajos de Pantanos (Familia Scirtidae)Observ.
onotoleDescripción
A larvae from a puddle. Thanks to Давид from "Энтомологический клуб" Telegram channel for ID.
Fotos / Sonidos
Qué
Orden HelotialesObserv.
heelsplitterDescripción
Growing on a hardwood stick with another discomycete (observation 320068). All structures inamyloid. Excipulum composed of irregular brownish (in Melzer’s reagent) cells. Paraphyses filiform. Asci 8-spored, pleurorhynchous, inoperculate, unitunicate and clavate. Asci measured with Piximetre: 36.37 × 4.91 µm, 26.74 × 5.29 µm, 30.29 × 4.06 µm, 28.21 × 4.62 µm
Spores smooth, hyaline, non-septate and biguttulate. Spore measurements from Piximetre: (4.2) 4.5 – 5.3 (5.8) × (1.6) 1.7 – 2.1 (2.3) µm, Q = (2.1) 2.2 – 3.1 (3.2); N = 20, Me = 4.9 × 1.9 µm; Qe = 2.6
Individual spores: 4.67 × 2.19 µm, 4.73 × 1.78 µm, 5.04 × 2.00 µm, 4.85 × 2.12 µm, 4.69 × 2.02 µm, 4.78 × 1.70 µm, 5.84 × 2.28 µm, 5.23 × 1.65 µm, 4.63 × 1.84 µm, 5.19 × 2.06 µm, 4.87 × 1.64 µm, 4.54 × 1.72 µm, 4.90 × 2.03 µm, 4.66 × 1.80 µm, 4.39 × 2.00 µm, 4.60 × 1.75 µm, 5.29 × 1.70 µm, 5.59 × 1.79 µm, 4.23 × 2.04 µm, 4.62 × 1.57 µm
Fotos / Sonidos
Observ.
crseaquistDescripción
Gathered dry leaves on 2024-02-03 and stored in water.
Fotos / Sonidos
Qué
Metopus esObserv.
peptolabDescripción
Metopus species most consistent with Metopus es (Müller, 1776) Lauterborn, 1916 from the acidic freshwater kettle pond Chatfield's Hole. Culture 2.5 months old, fed with boiled wheat seed. Imaged in Nomarski DIC on Olympus BH2S using SPlan 100 1.25 oil immersion with oiled condenser and Splanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The population has most features consistent with Metopus es except for the thick hyaline striated cortex with perpendicularly arranged rod-shaped extrusomes (or ? ectosymbionts). Such a thick hyaline cortex is not described for M. es but is a feature of M. fuscus and M. ovalis, but these species have markedly different characters from my population. The population here lacks the S-shaped body of M. es, however Bourland et describe loss of this feature in older cultures: " Specimens from older cultures usually broader, lack sigmoid shape, less twisted on long axis,more acuminate posteriorly" (1). Indeed, my culture was 2.5 months old when imaged and had been fed with boiled wheat seed.
The cells measure 140 um in average length. Cells hyaline, dark at anterior pole due to cytoplasmic granule aggregate. Shape usually elongate, slightly sigmoid,distinctly twisted anteriorly and often in posterior third. Specimens from older cultures usually broader, lack sigmoid shape, less twisted on long axis. Preoral dome slightly convex, usually over hangs left margin, less commonly overhangs right margin, inclined 45◦ to long axis, brim rounded. Body posterior to preoral dome ellipsoidal in cross-section, often flattened in posterior part. Macronucleus ovoid and extends only slightly into preoral dome; chromatin coarsely granular, scattered 2.5–5 m nucleoli; no localized perinuclear endobionts. Micronucleus spherical in depression in midportion of macronucleus. Cytopyge not visible. Contractile vacuole terminal, obconical, excretory pore not observed. Satellite vacuoles seen, ? food vacuoles or derived from channels depicted by Kreutz (2). Cortex flexible, appears thick and hyaline with a perpendicular regular row of rod-shaped subpellicular structures ? extrusomes vs symbionts. Prominent kinetal furrows with interkinetal cortical granules (mucocysts) ellipsoidal to globular, i.e., about 1.5 m-long in vivo, arranged in about eight ordinary interkinetal rows; colorless. Anterior pole granule aggregate comprised of densely packed 1.0–1.5 m dark brown spherical granules. Cytoplasmic endosymbionts rod-shaped, conspicuous in vivo under oil immersion. Food vacuoles up to 15 m in diameter. Ordinary somatic cilia about 10 m long in vivo, perizonal stripe cilia about 15 m in vivo, beat in metachronal waves; slightly elongated, i.e., about 13 m-long posterior cilia, inconspicuous in vivo, Swimming pace moderate; rotates on long axis.
- Redescription and molecular phylogeny of the type species for two main metopid genera, Metopus es (Müller, 1776) Lauterborn, 1916 and Brachonella contorta (Levander, 1894) Jankowski, 1964 (Metopida, Ciliophora), based on broad geographic sampling. William Bourland, Johana Rotterova and Ivan Čepička. European Journal of Protistology Volume 59, June 2017, Pages 133-154
- https://realmicrolife.com/metopus-es/
Fotos / Sonidos
Observ.
crseaquistDescripción
Water sample (freshwater) was taken on 2023-04-09 using a turkey baster.
Fotos / Sonidos
Observ.
peptolabDescripción
Stentor coeruleus Ehrenberg, 1830, showing a rare and unusual bicoloration which is likely environmentally produced from a sample of the riverside benthos of the freshwater portion of the estuarine river Peconic River. The sample was fed with boiled wheat seed. Imaged in Nomarski DIC (and brightfield at 40x and 100 x) on Olympus BH2S using Splan 4, Splan 10, SPlanapo 20 0.70, Splanapo 40 0.95, and SPlan 100 1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+.
The cells so far showed only swimming forms and measure from 520 up to 800 um in length; I have not seen them anchor and fully extend in the manner of S. coeruleus. This size range is rather small for Stentor coeruleus. The spiral of the oral pit appears dark at low magnification and red with higher magnification and stands out starkly from the turquois blue body. At high magnification, one can see mainly turquoise-colored pigmented pellicular granules with fewer smaller red granules between the somatic kineties but red granules are strikingly concentrated in the buccal spiral pit.
I first considered S. introversus Tartar 1958 based on the mentioned close resemblence to S. coeruleus in most characters- turquois blue color without symbiont algae, moniliform macronculeus, smaller size (1,2). My misdiagnosis was largely based on a paper by Kim et al 2020 (3) who redescribed a brackish population which they called S. introversus based largely on the presence of two colors of granules, turquoise blue (somatic) and red (3). However, this feature is not mentioned anywhere with regard to S. introversus. In fact Foissner et al describe S. introversus TARTAR 1958: "which is described in the USA and is identical in granulation and nuclear apparatus, is slightly smaller (up to 450 um), has a brown-yellow cytoplasm and everts its front end characteristically during contraction" (1). Perhaps more importantly, Kim et al 2020 (3) did not illustrate the main defining character of S. introversus in their population as defined by Tartar 1958 (4): the eversion of the buccal field, and that "border of recurved ectoplasm" which he calls "somewhat scalloped". Indeed, the buccal field of my population is that of garden variety S. coeruleus.
So, how to resolve these differential diagnostic discrepancies. Foissner and Wölfl 1994 included S. introversus in their review of the genus: "Stentor introversus Tartar, 1958. This species matches most characters of S.coeruleus. It is, however, smaller [450 um, up to 580 um, according to Jones (1974); weak character] and the entire zone of adoral membranelles cannot only contract, but also retract. We recognize this form as a distinct species because heteroplastic graft combinations between S.introversus and S.coeruleus did not survive (Tartar, 1958), and because Tartar was an expert on S.coeruleus making it unlikely that he was mistaken" (2). Kim et al found Stentor inversus in Korea, ostensibly the first report of the species since Tartar described it in 1958. "Stentor introversus Tartar, 1958 Diagnosis. Extended body size in vivo 500-600×200 250 μm and trumpet-shaped, contracted body size 250 305×200-250 μm in vivo (n=10) and pouch-shaped with retracted buccal field; cytoplasm colorless; 2 kinds of cortical granules densely arranged in between somatic kineties, larger granules turquoise and 0.4-0.5 μm in diameter, smaller granules reddish and 0.2-0.3 μm in diameter; 250 adoral membranelles; 60-73 somatic kineties; 16-20 buccal kineties including 1 peristomial kinety; moniliform macronucleus with 9-14 nodules. Distribution. U.S.A. and Korea. Remarks. Stentor introversus was not reported since the original description by Tartar (1958) from USA. It differs from the most similar species, S. coeruleus (Pallas, 1766) Ehrenberg, 1831, in the extended body size (500-600 μm vs. 1-2 mm), buccal field retracted when contracted (present vs. absent), and the number of types of cortical granules (2 vs. 1) (Foissner et al., 1992; Foissner and Wölfl, 1994) (3).
In evaluating my diagnosis of this population as S. introversus, Bruce Taylor made me aware of some interesting research on S. coeruleus by Tartar 1972 (5), who Foissner acknowledges as an " expert on Stentor coeruleus" (2). In Tartar's experiments, "after a few days in caffeine solution the dark blue-green ciliate Stentor coeruleus loses all visible pigmentation. The cells remain viable and transparent if the solution is then diluted. In time, however, a light pink color is regenerated in maintenance concentration of the drug. In caffeine-free medium the major dark green pigment is regenerated" (5). Tartar feels that the pink coloration was present all the while: "at first colorless, the animals acquired a light pink color, sometimes two or three days after bleaching or sometimes only three or four weeks later, depending on the strain. That this pigment was regenerated and not a new pigment developed under the influence of caffeine is indicated by the fact that at weaker bleaching concentrations of the drug the slower decoloration removed the green but left a pink color from the start, as if a native pink pigment had been unmasked by loss of the green one" (5).
The presence of two native pigments, pink-red and blue, in S. coeruleus is also supported by the work of Moller 1962 (6). Tartar 1972 (5) pointed out: "These two pigments, now demonstrable by separate regeneration of the pink, probably are those discovered by Moller 1962 using extraction procedures on S. coeruleus: a pink one he called stentorin-1 which was alcohol soluble, and a predominating blue-green pigment - stentorin-2, not extractable with alcohol" (5).
So my population is almost certainly an environmentally induced phenotype of Stentor coeruleus which, due to some unknown environmental stimulus, has developed a strikingly visible pink-red coloration of the oral spiral while retaining its usual predominant turquoise granulation elsewhere. The presence of two different colored granules in S. introversus sensu Kim et al 2020 is also explainable in this way. While my population of S. coeruleus is smaller, so far observed at 540-800 um, I have not yet observed sessile feeding forms which may well reach the 1-2 mm length reported for S. coeruleus.
- Foissner,W., Berger,H. and Kohmann,F. (1992) Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems-Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, May 1992, pp. 351-4
- Revision of the genus Stentor Oken (Protozoa, Ciliophora) and description of S.araucanus nov. spec. from South American lakes. W.Foissner and S.Wölfl. Journal of Plankton Research Vol.l6 no.3 pp.255-289. 1994
- Taxonomy of 16 indigenous ciliate species (Protozoa, Ciliophora) from South Korea. Ji Hye Kim, Atef Omar, Ji Hye Moon and Jae-Ho Jung. Journal of Species Research 9(4):427-442, 2020
- Tartar, V. (1958) Stentor introversus, n. sp. J. Protozool., 5, 93-95.
- Caffeine bleaching of Stentor coeruleus. Vance Tartar. Journal of Experimental Zoology Volume181, Issue 2 August 1972 pp 245-25.
- Moller, K. M. 1962 On the nature of stentorin. C. R. Lab. Carsberg, 32: 471-498.
Fotos / Sonidos
Observ.
davidfbirdDescripción
Second observation of S. niagarae, this time to show the presence of chitin fibrils, just like its close relative in the Stephanodiscaceae, Cyclotella (or Stephanocyclus). The second shot shows a full fibril. It is twice as long as the cell is wide. I can't find literature reports of fibrils on this genus, but maybe the whole family has them.
Fotos / Sonidos
Qué
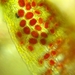
Vida (Vida)Observ.
abelkinserDescripción
These red "spores" were found all over a small moss growing on vertical sandstone. Can't do better than life. Maybe a lichen? I don't believe it's part of the moss. Any help is appreciated.
Fotos / Sonidos
Qué
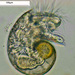
Animales (Reino Animalia)Observ.
kenkDescripción
Appears to be a larval something. Marine specimen
Fotos / Sonidos
Qué
Bacterias (Reino Bacteria)Observ.
crseaquistDescripción
Sample taken from Stock Tank, 2023-07-27.
Fotos / Sonidos
Qué
Vida (Vida)Observ.
someplantDescripción
Magnification of photos: 100×, 100×, 100×, 200×, 200×, 200×, 400×, 400×, 400×, 400×, 600×, 600×, 600×
Habitat: water and organic matter floating, in a salt marsh. Salinity unknown, but probably brackish.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 100× is 600 µm.
Fotos / Sonidos
Qué
Rabditóforos (Subfilo Rhabditophora)Observ.
mnold1Descripción
Mag. 400x
3 specimens imaged at different focal lengths. I'm inferring a relationship among them; a maturation process seems likely. Plant? Protozoan? Insect? Is this a stalked egg, similar to that of a Lacewing, but much smaller? (...the aftermath of a microbial wine tasting party? :o)
- A water sample was taken on 4/26/2023, from the shore of a Pine Swamp impoundment, using a 10µ dip net to enrich for microbes. Air temp. 58°F.