Fotos / Sonidos
Qué
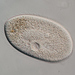
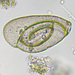
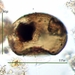
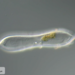
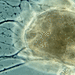
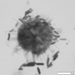
Urocentrum turboObserv.
peptolabDescripción
Transverse fission and conjugation in Urocentrum turbo. Imaged in Nomarski DIC using Olympus BH2S under SPlan 40x objective plus phone cropping. From freshwater portion of Peconic River.
Urocentrum Nitzsch, 1827.
Class Oligohymenophora: Subclass Hymenostomata: Order Hymenostomatida
Body cylindrical and ventrally slightly flattened; constricted at the middle; 2 broad girdles of cilia and 1 eccentric posterior tuft; a zone of short cilia in the constricted area; buccal cavity subequatorial with 1 membrane and 2 short undulating membranelles; in the postoral area, a longitudinal zone with small cilia; macronucleus horseshoe-shaped and posteriorly located; a single micronucleus; contractile vacuole terminal, and with 8 collecting canals; large number of trichocysts all over the body.
Fotos / Sonidos
Qué
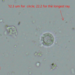
Bodo saltansObserv.
closterium_mysteriumDescripción
A water sample was taken from a vase with flowers from a cut on a flower stem at room temperature.
Video: https://youtu.be/OEdqZ8yh4Ec
Fotos / Sonidos
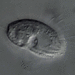
Observ.
peptolabDescripción
Unidentified frontoniid? from the sulfidic fine sand superficial intertidal benthos of marine estuary Acabonac Harbor at Landing Lane. The cells measure 200 um in length and resemble a Frontonia . The cytoplasm is quite vacuolated and there seems to be one dominant contractile vacuole in the center of the cell, though there are smaller vacuoles devoid of food. Only in some individuals can I see a ramifying network of collecting channels which I cannot really count or trace most of them to the food vacuole. Today I managed to image the CV pores and there are two. Other individuals show no collecting channels. There is a central ellipsoidal macronucleus and two spherical micronuclei, maybe sometimes more. But the most striking and unusual feature is the presence of a prominent cytopharyngeal basket of thick rods beneath the usual penniculine oral apparatus. It was surprizing because at first, my focus was on what looked like a cyrtos- as the organism moved I focused through the oral area and I noticed this quite prominent conical cytopharyngeal basket of rods which led me at times to wonder if I was looking at a cyrtophorid as the focus changed from the peniculine oral appsratus to the underlying cyrtophorid-like basket of rods. I cannot see three peniculi, I see two rows that sesmble peniculi but they look quite different from the usual 3 peniculi. These appear coninuous with a large undulating stucture. I have included some pictures which I labeled 3 peniculi but I doubt this and think I am incorrect in these pictures. The postoral suture is quite prominent, preoral suture less so. The ciliate feeds on diatoms though the one dominant food vacuole contains other food material.
I searched around my library of PDF's and searched online and in Xu et al 2018 I came across the family Clathrostomatidae which "has slightly differentiated oral polykinetid as multiple dikinetid rows and nematodesmata around cytopharynx (Small & Lynn, 1985)". I cannot find very much about it. I saw Kahl's three Clathrostoma species, all fresh water. I also came across mention of a Clathrostoma salina online but there is no descriptor or year described and I can find nothing. I suspect this might be the elusive Clathrostoma salina or perhaps another undescribed estuarine form of the genus. Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 40 0.95 and SPlan 100 1.25 oil objectives plus variable phone cropping on Samsung Galaxy S9+.
Fotos / Sonidos
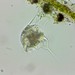
Observ.
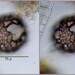
mnold1Descripción
Mag. 400x
A pond-side water sample taken on 02/24/2024 using a small sample bottled attached to an extension pole Air temp 42F (no ice at the shore).
Small, stalked zooids(?) ranging from 12 to 22µ in length (and perhaps smaller, based on some nearby, tiny epiphytic objects). Irregularly shaped, no loricae(?). All are epiphytic on a strand of Tabellaria. No movement observed. No flagella, no aperture or operculum. Tiny sessilida? Help!
Fotos / Sonidos
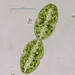
Observ.
mnold1Descripción
mag. 400x
- A pond-side water/periphyton sample was taken 04/22/2024 using a small sample bottled attached to an extension pole. Air temp 61F.
Similar to Gloeocapsa pictured here https://www.flickr.com/photos/johngiez-/4465267701.
Not certain about the ID. No multi-laminations are visible in the sheaths. Colony appears to be an aggregate of multiple sheathed units, most often a unit appears to contain 2 cells. (Compare views at different focal plans in the second image (a composite). When in focus, the cells appear to have a granular/punctate surface; see the last image.
Qué
Plantas (Reino Plantae)Observ.
mnold1Descripción
Mag. 400x
- A water sample taken on 03/31/2024, from a small vernal stream in a wooded, rocky area (rocks = New England stone wall-sized), using a small sample bottled attached to an extension pole. Air temp 46F.
Pollen grain? Dark brown color. Core of each lobe contains green tissue or cells. Couldn't find a match among plant pollen or moss and fern spores. Help, please!
Fotos / Sonidos
Observ.
peptolabDescripción
Heterometopus palaeformis Kahl 1927 Foissner 2016 from decomposing wheat seeds placed in a sample of benthos of the acidic freshwater kettle pond Chatfield's Hole. Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 40 0.95 objective plus variable phone camera cropping on Samsung Galaxy S9+. The cells measure from 90-100 um in length. The body is slender and elongated with roughly parallel sides but occasional individuals are plumper. The peristome roughly 30 % of the cell length and is straight not twisted. The adoral zone is ventrally located and straight, not in the right lateral position and convex as seen in Metopus. The preoral dome is small and does not overhang the body. There is an elongate sausage-shaped macronucleus with a somewhat wavy border in the anterior half of the cell. There is a spherical micronucleus usually overlapping the edge of the macronucleus. There are no caudal cilia or setae. There is a terminal contractile vacuole.
This morphology is that of the common freshwater ciliate Metopus palaeformis. Kahl 1927 as redescribed by Foissner et al 2002 (1). However, Foissner 2016 (2) transferred M. palaeformis to the genus Heterometopus. Foissner writes: " The most conspicuous and diagnostically important features of Heterometopus are the J-shaped zone of adoral polykinetids and the slender, cylindroid body" (2). My population (n=6) differs somewhat in that the long ellipsoid maconucleus is quite anteriorly placed often beginning at the anterior 10% of the cell and seldom extending past the equator. This is in contrast to the postoral position described by Foissner et al 2002 (1). But Esteban et al among others note that the morphology of M. palaeformis is quite variable (3).
From Foissner et al 2002 (1) "Improved diagnosis: Size about 100 x 25 um. Elongate ellipsoidal, hardly spiralized, preoral dome inconspicuous because only slightly curved and flat. Macronucleus postoral in middle body third, oblong. Cortical granules in loosely spaced rows, minute, strongly refractive. All somatic cilia of similar length, arranged in an average of 18 meridional rows, 5 modified to a perizonal stripe about half as long as adoral zone composed of 20 membranelles on average" (1).
"Description of Madagascan neotype population: Size 80-120 x 15-30 um in vivo, in other populations 70-200 x 8-31 um (ESTEBAN et al. 1995); length:width ratio 3.7-5.6:1, on average 4.3:1 in vivo. Shape inconspicuous, that is, elongate ellipsoidal and only indistinctly spiralized and flattened, preoral dome comparatively inconspicuous because only slightly curved and flat. Macronucleus postoral in middle third of cell, globular to very oblong (6:1) and more or less distinctly tortuous. Micronucleus attached to macronucleus at varying positions, about 4 um across in vivo. Contractile vacuole in posterior body end, excretory pore not recognizable. Cortex distinctly ribbed along ciliary rows; cortical granules conspicuous, although colourless and only 0.2-0.3 um across, because strongly refractive and in distinct, occasionally zigzagging rows within and between kineties. Cytoplasm colourless, packed with granules 1-2 um across and 3-5 um long rods,likely methanogenic bacteria, which intensely impregnate with protargol. Likely feeds on bacteria. Movement inconspicuous. Cilia about 12 um long in vivo, no elongated caudal cilia, paired only in dome area, anterior basal body of dikinetids barren and slightly smaller in other regions; arranged in an average of 18 equidistant, almost meridional rows, leaving blank posterior pole centre. Perizonal ciliary stripe of usual structure, short, that is, about half as long as adoral zone of membranelles. Adoral zone inconspicuous because occupying merely one third of body length, only slightly spiralized, and membranellar bases only 4 um long. Paroral membrane short and inconspicuous" (1).
"Occurrence and ecology: Metopus palaeformis is a common freshwater ciliate and rare in terrestrial habitats, where M. hasei is much more frequent. It is an obligate anaerobe lacking conventional mitochondria, like the congeners (Esteban et. al. 1995). Reliable records are known from Europe (Esteban et. al. 1995, KAHI 1932), Namibia and Madagascar. Identification and comparison with related species: Our observations match the original description (Khal 1927a, 1932) and add significant data on the infraciliature. Thus, identification is beyond reasonable doubts, and the population can serve as a neotype. Metopus palaeformis is highly similar to M. hasei, as redescribed by Foissner & Agatha (1999), except for the lacking caudal cilia. Indeed, this is the sole reliable difference, especially when the considerable variation both species exhibit is taken into account (Esteban et al. 1995, Foissner & Agatha 1999). A minor difference concerns the preoral dome, which is more distinctly curved in M. hasei than in M. palaeformis" (1).
"Metopus palaeformis has been kept in culture and studied ecologically for several years by Finlay and co-workers (for a brief review, see Esteban et al. 1995). Detailed morphological data were not provided, although Esteban et al. (1995) (3) documented various cytological features and the morphological variation by excellent micrographs. The strong variation was not seen in the specimens from the non-flooded Petri dish culture and seems to be related mainly to starvation. However, KAHL (1932) observed similar variation in nature and distinguished a forma typica, ovalis, and, attenuatus. Based on the data available, Esteban et al. (1995) suggest the following synonymy, with which we largely agree: M. hyalinus KAHL, 1927a; M. rostratus Kahl, 1927a; M. tenuis KAHL, I927a; and Tesnospira alba Jankowski ,1964b- (for the last two the synonymy is doubtful in our opinion)" (1).
"Heterometopus differs from Metopus s. str. mainly by the shape and location of the adoral zone of polykinetids, a feature used to split Metopus s. l. into several subgenera, especially by Jankowski (1964a,b, 2007). This has been widely acknowledged (Bourland and Wendell 2014; Foissner 2016; Lynn 2008) and the subgenera raised to genera. In Heterometopus, the J-shaped adoral zone extends slightly obliquely on the ventral side and makes a sharp proximal turn when it enters the cell. Indeed, this pattern is highly similar to those found in various heterotrichs, for instance, Blepharisma and Pseudoblepharisma. In Metopus s. str., the adoral zone extends ventrally and right laterally becoming more or less sigmoid and slightly to distinctly convex on the right body side. This causes a very different appearance when Metopus and Heterometopus are seen side by side. The most conspicuous and diagnostically important features of Heterometopus are the J-shaped zone of adoral polykinetids and the slender, cylindroid body" (2).
- Soil Ciliates (Protozoa) Ciliophora) from Namibia (Southwest Africa), with Emphasis on Two Contrasting Environments, the Etosha Region and the Namib Desert PART I: Text and Line Drawings. Wilhelm Foissner Sabine Agatha and Helmut Berger. Denisia 5 June 2002 , pp. 885-9.
- Heterometopus meisterfeldi nov. gen., nov. spec. (Protozoa, Ciliophora), a new metopid from Australia. Wilhelm Foissner. European Journal of Protistology Volume 55, Part B, September 2016, Pages 118-127
- Esteban, G., Fenchel, T., Finlay, B., 1995. Diversity of free-living morphospecies in the ciliate genus Metopus. Arch. Protistenk. 146, 137–164.
Observ.
davidfbirdDescripción
From the foam accumulated at rock barriers in the stream, another kind of aquatic hyphomycete conidia. Cells are 2-4 x 1-1.5 µm. I'm not sure of the identification - going out on a limb.
Qué
Carpintero Velloso-Menor (Dryobates pubescens)Observ.
mnold1Descripción
Right woodpecker = Downy
Hairy and Downy were seconds apart on the same feeder. Assembled images in a single composite to emphasize the size difference.
Fotos / Sonidos
Observ.
davidfbirdDescripción
From a sample of periphyton collected in a small stream that crosses the trail. Cell length 113 µm. Large central macronucleus and prominent micronucleus. F. acuminata?
Qué
Género EunotiaObserv.
mnold1Descripción
Mag. 400x
- A pond-side water sample taken on 02/24/2024 using a small sample bottled attached to an extension pole Air temp 42F (no ice at the shore).
A larger specimen was previously observed, https://www.inaturalist.org/observations/158455990. Both appear similar to Eunotia mayamae as seen here https://phytokeys.pensoft.net/article/23806/zoom/fig/199/, an excerpt from https://phytokeys.pensoft.net/articles.php?id=23806.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample taken on 02/24/2024 using a small sample bottled attached to an extension pole Air temp 42F (no ice at the shore).
Stalked, loricate ciliate in the role of an epizoite; it is attached in the urosome/seta region of a copepod (tail area). The copepod was very active, whipping the Cothurnia sp., and its associated epizootic alga, in sweeping arcs... a testament to the strength of the attachments involved. The last image (replicated in composite image 2) may give a hint of the nature of the pellicle of the zooid; see the horizontal striations at the base of the zooid. I was unable to capture a good video of this critter and not thoughtful enough to photograph the entire copepod with its Cothurnia passenger.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F. (sample has been sitting on a cool, west-facing window ledge since 1/6/24)
Small, smooth Arcella, partial ventral view (50µ wide, 13µ aperture). For the purpose of size comparison, the last composite image includes recently observed Arcella species from the same water sample, all normalized to the same scale.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample taken on 02/24/2024 using a small sample bottled attached to an extension pole Air temp 42F (no ice at the shore).
The 3 images derive from 3 slightly different focal planes (looking for flagella). Initially thought this may be Apiocystis brauniana, which has been observed in the area. However the tubular containment of the cells prompted more looking. Palmodictyon varium seems a perfect fit, given its tube-like sheath surrounding the cells and the undulate sheath margin (bulging round cells), characteristics that correspond well with images at -algaebase.org-, https://www.algaebase.org/search/images/detail/?img_id=19805. (also see http://cfb.unh.edu/phycokey/Choices/Chlorophyceae/colonies/colonies_not_flagellated/PALMODICTYON/Palmodictyon_key.htm)
Observ.
joseph92Descripción
The high-pitched ringing gradually rises in volume as other frogs join in and start doing more of a trill-type call. Not sure which species is responsible.
Fotos / Sonidos
Qué
Coyotes, Lobos, Chacales Y Perros (Género Canis)Observ.
mnold1Descripción
Individual track ~2.25 - 2.5"
Claw marks prominent.
Prints were related by size, direction and the absence of other tracks.
The first track looks like domestic dog, though there are no loose dogs in the extended neighborhood.
The second track, which I believe was made by the same animal, looks more fox or cayote in shape. The last photo captures a paused position (I think) at the corner of a building. Please help with an ID if able. Thanks!
Fotos / Sonidos
Qué
Ardillas Y Parientes (Familia Sciuridae)Observ.
mnold1Descripción
Individual track length ~1.5"
Claw marks prominent.
Track Pattern: Front and hind tracks approximately parallel to form a single track set. The next set appears 18" to 14" from the previous set. This pattern repeated for 5 sets of tracks, see composite photo.
Not sure if this is a hopping or galloping pattern. Red fox are common in the area, as are squirrels, racoons, skunks, etc. Please help ID if you can. Thank you!
Fotos / Sonidos
Observ.
peptolabDescripción
Mastigamoeba species from the intertidal benthos of marine estuary Acabonac Harbopr at Louse Point imaged in Nomarski DIC on Olympus BH2 under SPlan 40x objective plus variable phone cropping on Samsung Galaxy S9+. Folllowing discussion is from: https://de.wikipedia.org/wiki/Mastigamoeba
Mastigamoeba is characterized as a genus of unicellular organisms characterized by an amoeboid (polymorphic) body with hyaline (transparent) cytoplasm and a flagella (amoeboid flagellates). Currently, the World Register of Marine Species lists only two marine species: M. pachyderma Skuja, 1948 and M. schizophrenia Simpson, Bernard, Fenchel & Patterson, 1997.
Due to its similarity to genera such as Mastigella and Mastigina, the genus Mastigamoeba was specified in 1891 to include only organisms with the following characteristics: amoeboid flagellates with hyaline cytoplasm, a direct connection between flagella and nucleus, lateral pseudopodia (pseudopods) and an elongated nucleus. However, the members of the genus change between different morphologies during their life cycle and can exist not only as amoeboid flagellates but also as unflagellated (aflagellate) amoebae, as multinucleated amoebas and as cysts.
During the 20th century, hundreds of species were described under the genus Mastigamoeba solely on the basis of external morphological features. However, recent research on their life cycle has shown that one and the same organism takes on many different morphologies (forms) over the course of its life, calling into question this large number of species described. Currently, there are about nine confirmed and distinguished species of Mastigamoeba, with many more being questioned (see below). Cavalier-Smith described the class Archamoebea in 1983 and included the order Mastigamoebida with the genus Mastigamoeba. Historically, amoeboid flagellates were included in the Pelobionta, with the Mastigamoebidae and the Pelomyxidae (genera Pelomyxa and Mastigella). The naming of the genus is controversial. The genera Mastigamoeba and Phreatamoeba are now considered synonymous by many researchers, although this is questioned by others.
The representatives of the Mastigamoeba are microoxic, i.e. they thrive in environments with low oxygen content (10–20%), e.g. in upper layers of mud or sand or on the sediment surface of shallow ponds. Some have also been found in sewage treatment plants. Such pelobionts are usually found worldwide, studies have confirmed their widespread occurrence in the temperate regions of Europe and North America. Typical habitats include habitats rich in organic matter. Among freshwater rivers and lakes, these organisms are most abundant in stagnant waters where oxygen-poor environments are common. Pelobionts are also found in marine environments. Although most pelobionts are free-living, some representatives are considered endobiotic, i.e. they survive only in the intestines of their hosts. These representatives are completely anoxic and thrive at low pH. They have been found in various vertebrates and invertebrate hosts, especially primates and dogs.
The length of the flagella ranges from 10 μm to 60 μm. In amoeboid locomotion with the help of the pseudopods, the species of clade A have a so-called uroid (also uropodium,typically mulberry-like structure at the rear end, which can appear rounded, rotten and hairy), while the types of clade B instead have a trailing pseudopodium. There is only a single flagellum (flagellum). This consists of the 9+ microtubule structure typical of eukaryotes. The flagellum apparatus consists of a single basal body from which the scourge originates. There is a microtubularcone, a cone-shaped structure that connects the flagellum apparatus directly to the cell nucleus. In the types of clade A, this cone is wide and originates at the base and lateral ends of the basal body. In the species of clade B, however, it is narrow and originates only at the base of this structure. The flagellum apparatus is arranged anteriorly and supports locomotion.
The outside of the cell is covered with a thin, unevenly distributed layer of organic, filamentous (threadlike) material. These threads run parallel to the cell and are 1 μm thick at their thickest point. The chemical composition of this extracellular shell is unknown. Some Mastigamoeba species have spines that are irregularly distributed around the cell. These spines are hollow, and their composition is also unknown. The organic layer sometimes contains prokaryotic symbionts of unknown identity; the exact relationship between the Mastigamoeba sp. and this symbiont is unknown (as of 2011). Another feature of the genus Mastigamoeba is the lack of a Golgi apparatus (dictyosome). However, its central functions are taken over by related elements in the endomembrane system – the endoplasmic reticulum contains some bundled structures and various vesicles that perform the core functions of a Golgi dictyosome. Not all Archamoebae have peroxisomes present. However, studies have shown that some Mastigamoeba species contain at least peroxisomal proteins.
The Archamoebae are all amitochondria, i.e. they have no typical, true mitochondria. The mitochondria in the mastigamoeba have been reduced (in the course of evolution) or transformed into forms that still retain some mitochondrial functions or have altered functions. These are commonly referred to as mitochondria-related organelles (MROs). For example, species like M. balamuthi have MROs called hydrogenosomes. Hydrogenosomes have arisen from mitochondria due to the loss of aerobic life stages. The hydrogenosomes have lost their former genome and the electron transport chain in the course of evolution. However, they continue to be used for ATP production by partial anaerobic oxidation of pyruvate and produce hydrogen gas as a by-product. The biosynthesis of iron-sulfur clusters has passed into a cytosolic function through lateral gene transfer (from the MRO genome to the nuclear genome) and is no longer carried out in the MROs. Other species have reduced mitochondrial organelles called mitosomes. These MROs have been reduced to such an extent that their only function is the biosynthesis of iron-sulfur clusters. They no longer have a function in energy metabolism, so these organisms must obtain their energy in other ways. To compensate for the loss of their own ATP production, these amitochondrial organisms have acquired the ability to import ATP from a host or symbiotic partner.
Several cellular phenotypes are known from many members of the Archamoebae, e.g. (mononucleated) amoeba, multinucleated amoeba, cyst and flagellate. The cyst stage has a single nucleus and is filled with granules and surrounded by a wall of unknown composition (as of 1986). The most important trophic form (growth form, as opposed to resting form) of Mastigamoeba is a mononuclear amoeboid flagellate. However, some species show (at least in some phases of life) a multinucleated morphology: M. schizophrenia has up to 10 nuclei in the multinucleated stage. In M. balamuthi, the predominant trophic form is that of a multinucleated organism that can have up to 46 nuclei. Propagation occurs by mitosis and subsequent budding. In the multinucleated form, this usually results in an unequal number of nuclei in the daughter cells.
Fotos / Sonidos
Observ.
douchDescripción
Bright-field microscopy. Between barbs, on internal surface of detached feather (Cacatua galerita galerita / C. tenuirostris / C. sanguinea sanguinea).
Image 1: body
Image 2: anterior; upper focal-plane
Image 3: posterior; lower focal-plane
Image 4: anterior; upper focal-plane
Image 5: posterior; lower focal-plane
Fotos / Sonidos
Qué
Arcella vulgarisObserv.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Smooth dome shape with a turned-up brim (i.e., boarder of the base, like the brim of a hat). The aperture, measured in the first photo, is 30µ. The occupant of the test is not alive. For more information and images, see https://arcella.nl/arcella-vulgaris/.
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Single specimen. Striae density near center, 8/10µ. As seen here on iNaturalist and here https://diatoms.org/species/gomphonema_acuminatum.
Fotos / Sonidos
Qué
Vida (Vida)Observ.
mnold1Descripción
mag. 400x
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Looks like the test of amoeboid, Arcella. My wishful eye sees Arcella rotunda var. alta, or something very close. However, this specimen is too large and the texture of the test surface seems wrong (too smooth and no tessellation of tiny hexagons; though some images show a dispersion of small, crater-like features of irregular size). Question remains: Arcella or debris. (Image of A. rotunda var. alta., https://arcella.nl/arcella-rotundata/.
Fotos / Sonidos
Observ.
peptolabDescripción
A peculiar ciliate, probably a spathidiid, with features closely resembling Apertospahula okteme Yildiz 2018 found in a four month-old sample of the coarse sand intertidal benthos of marine estuary Acabonac Harbor at Louse Point launching ramp. Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 40 0.95 objective plus variable phone camera cropping on Samsung Galaxy S9+.
The ciliate measures 66 um in length. It has a prominent somewhat bulbous perhaps open oral bulge associated with an eccentric oral opening and an aggregate of refractive granules. There is an oblong macronucleus in the posterior half of the cell and a terminal contractile vacuole. The cytoplasm is filled with digestive vacuoles.
The genus Apertospathula comprises ten species most of which are terrestrial. The only aquatic species described thus far is A. okteme which was found in bottom sediments of a marina of Lake Van in Turkey with salinity of 19%. My specimen resembles A. okteme in its saline sediment biotope, claviform shape, size, anterior aggregate of refractive granules, and oblong macronucleus. The oral bulge of my specimen is more promnent and bulbous than A. okteme and the anterior aggregate of refractive granules is smaller than that of A. oketeme where it fills the anterior 1/3 to 1/2 of the cell.
I feel that this specimen might be an as yet undescribed marine apertospathulid species or perhaps it belongs to another spathidiid genus. The genus Apertospathula features a ventrally open, loop-like oral bulge which I think we can see in this specimen.
Morphology and phylogeny of Apertospathula oktemae n. sp. (Ciliophora, Haptoria, Spathidiida) from Lake Van, Turkey. Ismail Yıldız. European Journal of Protistology 66 (2018) 1–8
Fotos / Sonidos
Observ.
igorvianagDescripción
Ceratomyxa sp. extraído de um peixe amazônico.
Visão microscópica de um parasita Ceratomyxa sp.
Fotos / Sonidos
Qué
Abaniquillo Pardo del Caribe (Anolis sagrei)Observ.
rubynature2000Descripción
1st - 3rd Nov 2023 images collection
Fotos / Sonidos
Qué
Gorrión Molinero (Passer montanus)Observ.
rubynature2000Lugar
Falta la ubicaciónFotos / Sonidos
Qué
Lepocinclis acusObserv.
mnold1Descripción
Mag. 400x (1), 200x (2)
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
Corresponds well with images of Euglena acus found here http://protist.i.hosei.ac.jp/PDB/Images/Mastigophora/Euglena/acus/acus2.html. Inventory: 1 flagellum, 1 eyespot, 2 paramylon granules (rod-shaped, starch storage bodies in the anterior section), discoidal chloroplasts with the appearance, in this specimen, of hexagonal panels in the outer membrane (these are most easily seen, in the central to posterior section, in the middle photo of the composite image and in the last image at lower magnification (but better depth of field).
Fotos / Sonidos
Observ.
amayakanDescripción
Cell size: approx. 4–5 µm
Site of collection: Katsurashima Ryokuchi south pond (a freshwater habitat), Sendai, Japan
Date of collection: June 5th, 2021
Weather: Sunny
Water temp.: 22.0°C
pH 6.7
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan).
Movie:
https://youtu.be/8969Rv2i6GA
https://youtu.be/fHdFtUfNSLM
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x (1), 200x (2,3)
- A pond-side water sample (retentate) was taken on 01/06/2024 using a 10µ dip net to enrich for microorganisms. (The shoreline had a layer of ice about 1/4" thick that needed to be breached in order to accommodate the dipnet. The retentate contained a lot of sediment due to the shallow depth and disturbance caused by the net.) Air temp 35F.
A euglenid with a single anterior flagellum, hyaline tail section, and a deeply ribbed pellicle (scales!, see comments) reminiscent of euglenoids Lepocinclis fusca and Phacus monilatus. Metabolic movement was not observed. Link to video https://youtu.be/GlnYtTwJ9YI.
Fotos / Sonidos
Observ.
tshahanDescripción
first waterbear! found in lichen/moss on rock wall in woods
Fotos / Sonidos
Observ.
peptolabLugar
PrivadoDescripción
Allogromia terricola (Leidy, 1874) syn. Gromia terricola Leidy, 1874 original description), syn. Gromia fluviatilis Dujardin. A terrestrial species of forminefera from an infusion prepared from the soil at the base of my compost heap.
Test spherical to subspherical; smooth or sparsely covered with siliceous particles; yellowish cytoplasm fills the test; aperture not seen; a large nucleus and numerous contractile vacuoles; filopodia long, often enveloping test; on aquatic plants, in moss or soil.
Envelope spheric or subspheric, seldom changing shape; protoplasm habitually covering the surface of the envelop. Pseudopodia numerous, anastomosing. Habitat aquatic plants. Measurements
90-250 um long.
The following discussion is adapted from the reference below.
Foraminifera are unicellular eukaryotes characterized by the presence of granuloreticulopodia and the possession of a membranous, agglutinated, or calcareous test, which is either monothalamous (single-chambered) or polythalamous (multi-chambered) (Loeblich and Tappan 1987). Within monothalamids some species like Reticulomyxa filosa are amoeboid naked forms.
Until 1859, foraminifera were only known from marine habitats, but that year Claparède and Lachmann described a monothalamid foraminifer, Lieberkuehnia wageneri, sampled from an unknown water body in Berlin. It had a smooth flexible test with an entosolenian tube that separated the main cytoplasm mass from the pseudopodial peduncle.
In 1886 Henri Blanc, a Swiss scientist, described another freshwater foraminifer, Gromia brunneri, which he had collected from the bottom of Lake Geneva. This single-chambered species had an agglutinated test, an organic layer covered and/or embedded with foreign, mainly non-organic, particles.
In subsequent years, Eugène Penard, another Swiss protozoologist, described four similar species Gromia gemma and G. squamosa (1899), G. linearis (1902) and G. saxicola (1905) from the same lake. He also described G. nigricans (1902), which he found not far from Lake Geneva in Mategnin and a marsh near Rouelbeau. Penard made permanent preparations of these foraminifera, which are still preserved and available in the Penard Collection of the Natural History Museum of Geneva (Switzerland).
In 1904, Ludwig Rhumbler erected the subfamily Allogromiinae for monothalamous foraminifera characterized by a more or less flexible organic test wall commonly with one or rarely two terminal apertures at either end of the test. He included all described freshwater species in this taxon. In a recent higher ranked classification of foraminifera based on molecular phylogenies (Pawlowski et al. 2013), monothalamous foraminifera were considered as a paraphyletic group that contains agglutinated and organic walled species as well as “naked” amoeboid species and environmental clades with unknown morphological affinities.
Traditionally the organic-walled foraminifera are called allogromiids. Most of them are distributed over a wide range of marine and brackish habitats (Gooday 2002). Freshwater allogromiids with an agglutinated test were originally placed in the genus Gromia by their discoverers, but as its type species G. oviformis is a filose marine species, Rhumbler (1904) transferred three species (G. squamosa, G. nigricans and G. linearis) to Rhynchogromia Rhumbler 1894. He further erected a new genus, Diplogromia, for the other two species having a double test wall: G. brunneri and G. gemma, although without designing a type species for the genus.
De Saedeleer (1934) revised Rhumbler’s classification leaving D. gemma in its genus and creating a new genus Allelogromia for the Rhynchogromia species with G. brunneri as type species. Deflandre (1953) erected the genus Penardogromia for G. linearis, with the argument that it had a homogenous agglutinated test with calcareous particles. Loeblich and Tappan (1960) argued that the classification of De Saedeleer was unacceptable, because G. brunneri had been fixed as the type of Diplogromia by subsequent designation of Cushman (1928). They created the genus Saedeleeria for G. gemma, transferring G. squamosa and G. nigricans also to Diplogromia, but without giving any supporting explanations. Another agglutinated allogromiid, Penardogromia palustris, was described by Thomas (1961) from a freshwater marsh near Bordeaux (France).
Beside these descriptions there have been some scattered records of agglutinated freshwater allogromiids over the years (Grospietsch, 1958, Hoogenraad and De Groot, 1940, Siemensma, 1982, Wailes, 1915; Meisterfeld pers. comm.; Clauss, unpublished) and some photomicrographs available online (Revello, 2015, Protist Information Server, 2016).
Leidy (1879) was the first who described an allogromiid foraminifer, Gromia terricola, from a terrestrial habit. He found this non-agglutinated species “among moist moss in the crevices of pavements, in shaded places, in the city of Philadelphia”. A similar terrestrial organic walled allogromiid Edaphoallogromia australica has been described by Meisterfeld et al. (2001).
Siemensman et al. Taxonomic revision of freshwater foraminifera with the description of two new agglutinated species and genera. European Journal of Protistology. Volume 60, August 2017, Pages 28-44 https://doi.org/10.1016/j.ejop.2017.05.006
Fotos / Sonidos
Observ.
davidfbirdDescripción
Small tardigrade hiding in the moss from along the trail. About 340 microns long. The colour in these photos is not representative of its natural transparence.
Fotos / Sonidos
Qué
Género VannellaObserv.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 10/14/2023 using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Small amoeboid organism. Often fan shape, narrow portion trailing, wide portion leading. The leading edge is transparent. Organellar material is concentrated in the trailing portion. This amoeboid looks very similar to images here https://arcella.nl/vannella-sp/. Photos 2 and 3 are for comparison with a larger, pseudopod-baring amoeboid. For a video, see https://youtu.be/yiZ1RHs2vOI.
Fotos / Sonidos
Observ.
woodilljDescripción
- sampled from standing water found in a metal bucket
- objectives (x10): image 1, 600x; image 2, 1000x
- Images 3 and 4 are animated GIFs. ~~Full video is on Vimeo.~~
- see also
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 10/14/2023 using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Relatively large Galeripora discoides, diameter 130µ; aperture 65µ; ratio of aperture to full diameter 0.5.
Reference: https://arcella.nl/galeripora-discoides/.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 10/14/2023 using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Not certain regarding the ID. Within the class Discosea is the candidate Oscillosignum distorta which matches drawings and habitat description presented in the 1985 "Illustrated Guide to the Protozoa", p183. The singular, pale green, granular organelle is the cell's nucleus, I think (labeled in photos).
For a video of this specimen see https://youtu.be/0PEXhiCqT2E.
Qué
Netzelia gramenObserv.
mnold1Descripción
Mag. 400x
Abandoned test. Trilobed aperture. As seen here https://arcella.nl/netzelia-gramen/.
- A pond-side water sample was taken on 06/10/2022 using a 10µ dip net to enrich for microbes. Air temp. 76°F.
Fotos / Sonidos
Qué
Orden HaptoridaObserv.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 10/14/2023 using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Relatively long ciliate (175µ).
Fotos / Sonidos
Observ.
peptolabDescripción
Thanks to Ryan Gawryluk for identifying this heliozoan as Ciliophrys species from biofilm that developed in a week old sample from the intertidal benthos of marine estuary Acabonac Harbor at Louse Point launching ramp. The cell body measures 15 um in diameter and has axiopods extending in three dimensions with granular kinetocysts. They taper slightly from base to tip. The long flagellum is held in a double figure 8 pattern. There are peripheral clumps of heterochromatin in the nucleus. These features are all indicative of Ciliophrys azurina Mirkujov and Patterson 2001 (1). In the first video we can see a bacterium being captured in a bag-like pseudopodium as depicted by Siemensma (2).
"Ciliophrys is a naked and heterotrophic pedinellid with either no stalk or a short stubby stalk (unpublished ultrastructural information). It is distinguished from other pedinellid taxa without plastids because the arms radiate from the whole cell surface, and, while in the heliozoan state, has weakly active flagellum held in a figure of 8 configuration. The cell may convert into an arm-less form at which time the flagellum becomes more active and the pseudopodia are withdrawn. These arm-less cells usually swim with the flagellum directed to the front. The fine, non-tapering axopods are supported by single triads of microtubules. As with actinophryids and other pedinellids, the interior ends of these axonemes are associated with nuclei. The composition of the genus was discussed by Larsen and Patterson (1990). We currently admit two species, and here add a third" (1).
"Ciliophrys azurina Patterson, sp. n. Diagnosis. Ciliophrys with tapering arms; nucleus with a central nucleolus and additional peripheral heterochromatin. Description. Cell 15 µm in diameter, with radiating arms with extrusomes. The single flagellum is held in front of swimming cells, and in non-swimming (feeding) cells the flagellum is held tightly curled, typically in a double “figure of 8”. The nucleus is large, prominent and has a nucleolus and clumps of material located around the inner face of the nuclear envelope. Observed consuming diatoms" (1).
"Remarks. Ciliophrys azurina can be distinguished from the other well described species in the genus, C. infusionum, by being considerably larger (15 µm vs 5 µm, although we note that C. infusionum has been reported as up to 20 µm long). More importantly, C. azurina can also be distinguished because the flagellum is longer and held in a double “figure of 8”, because the arms taper from base to tip, and because of the existence of peripheral clumps of heterochromatin in the nuclei. These two characters are held in common with Actinophrys - and there is especial similarity with Actinophrys pontica. We interpret the tapering arms and peripheral heterochromatin as being apomorphic characters for a previously unrecognised clade which includes C. azurina and the two genera of actinophryids and which we here refer to as the heliomonads" (1).
Imaged in Nomarski DIC using Olympus BH2S under SPlan 100x objective and oiled condenser plus variable phone camera cropping on Samsung Galaxy S9 + . One video includes some choanoflagellates just because they are so cute.
- Taxonomy and Phylogeny of Heliozoa. III. Actinophryids Kirill A. MIKRJUKOV and David J. PATTERSON. Acta Protozool. (2001) 40: 3-25.
- https://arcella.nl/ciliophrys-infusionum/
Fotos / Sonidos
Observ.
peptolabDescripción
Cyclidium glaucoma Möller from a biofilm that formed after a week on a sample of the coarse sand intertidal benthos of marine estuary Acabonac Harbor at Louse Point launching ramp. Imaged in Nomarski DIC on Olympus BH2S using SPlan 40x and 100x oil objective with oiled condenser plus variable phone camera cropping on Samsung Galaxy S9+. The cells measure from 20-24 um in length. The oral apparatus (undulating membrane) occupies from 1/2 up to 3/4 body length. There is a large spherical anterior macronucleus, the micronucleus is not well visualized. There is a long caudal cilium and some cells have a posterior dimple. There is an indistinct cone-shaped frontal plate. There is usually a terminal contractile vacuole and sometimes one or two additional CV in subterminal position are present.
The following is from Taxonomische und ökologische Revision der Ciliaten des Saprobiensytems Band lll Hymenostomata, Prostomatida, Nassulida. W. Foissner, H. Berger, F. Kohmann. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, Heft 1/94,548 pp.
Differential diagnosis
1) Size in vivo 14-30 x 6-16 um.
2) Shape barrel-shaped, in poorly fed specimens also spindle-shaped. Front end non-ciliated and slightly cone-shaped (frontal plate), rear end narrow to broadly rounded and, especially in starving cells, with a dimple from which the caudal cilia emerges. Not flattened.
3) Macronucleus spherical in the front half of the body. 1 spherical micronucleus.
4) Contractile vacuole terminal.
5) Resting extrusomes rod-shaped, very inconspicuous.
6) 10 (rarely 11) longitudinal ciliary rows. Cilia in the front half of the body mostly in pairs, at the rear end there is a caudal cilia approximately body-length and terminally curved.
7) Oral apparatus 1/2 body length. 3 very small adoral membranelles arranged one behind the other, right of which an L-shaped undulating membrane, the cilia of which are quite long and when resting. The animal forms a striking sail; detailed structure of the oral cilia only recognizable after silver impregnation, not necessary for the determination).
8) movement jumping and sliding; During the often long breaks the cilia are spread apart undulating membrane is set up like a sail and food is swirled in.
Possible confusion
The following Cyclidium species, some of which are very similar to one another, require a detailed description: C. citrullus (15-16 rows of cilia, --) taxonomy), C. elongatum SCHEWIAKOFF (hind end broadly truncated, oral apparatus about 3/4 body long, usually rests for a very long time and only drives a little back and forth when touched), -+ C. heptatrichum (middle section of body loosely ciliate, hind end with several slightly elongated cilia), C. oblongum BALD (40 pm; mouth on both sides of surrounded by a membrane; check), C. singulare (KAHL) (slender ovoid, contractile vacuole sub terminal, oral apparatus about 1/4 body long), C. versatile PENARD (swims rotating moderately quickly, with the long eyelashes trailing), C. litomesum STOKES (40 pm, dumbbell-shaped shape). -+ Ctedoctema acanthocryptum is significantly slimmer, the pellicle is usually notched and the contractile vacuole is further subterminal. -) Uronema nigricans is in the front third of the oral apparatus slightly dented and has a much more inconspicuous undulating membrane. -+ Pseudocohnilembus species are usually pointed egg-shaped and almost never rest. Features 2, 4, 7, 8 important.
Fotos / Sonidos
Observ.
pierrelfrDescripción
Collected on March 30, 2021. Length 100µ. Last photo is a gif.
Fotos / Sonidos
Qué
Género CodonellaObserv.
kenkDescripción
Time 3:30 PM
Weather: Clear
Air temp: 12C
Water Temp: 8.5C
Salinity: 34ppt.
Wind: 10-20 MPH, NNE
Tide: High tide 2.95
Secchi depth: 340cm
Fotos / Sonidos
Observ.
peptolabDescripción
Trepomonas steinii Klebs, 1892 from the northernmost saprobic edge benthos of the spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve. This sampling site is situated 250 meters from the edge of the Atlantic Ocean and is rich in decaying organic matter. Imaged in Nomarski DIC using Olympus BH2 under SPlan 40x objective plus variable phone cropping on Samsung Galaxy S9+.
Thanks to Ivan Čepička for identifying this observation. A diplomonad dance party. Today's slide from the northernmost saprobic edge benthos of spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve got richer and richer in diplomonad flagellates the longer the slide sat on the stage.
Cells were approximately 8 um – the smallest of all the species of the genus Trepomonas I found. The cells were slightly flattened and had a characteristic teardrop shape at first glance to triangular shape. The rear end was narrower than the front.
Diplomonad flagellates include the gnera Trepomonas and Hexamita. Most genera of diplomonads are parasites, and the few genera that are free-living are usually found in organically enriched (and usually anaerobic) sites. The cells are bilaterally symmetrical along their longitudinal axis. There are two anterior nuclei, and associated with each are four flagella which arise at the head of a groove in the body surface. The genera may be distinguished by the relative lengths of the flagella and by the flexibility of the bodies. In both genera, one flagellum of both quartets extends laterally from the head of the groove. The remainder lie within the groove, with those of the more pliable Trepomonas not extending beyond the posterior margin of the cell, as do those of Hexamita . These organisms may feed either by eating bacteria or by pinocytosis.
Free-Living Freshwater Protozoa: a Color Guide 1st Edition By D.J. Patterson. CRC Press Taylor and Francis Group. p 64.
Fotos / Sonidos
Observ.
peptolabDescripción
Trepomonas rotans Klebs, 1892 from the northernmost saprobic edge benthos of the spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve. This sampling site is situated 250 meters from the edge of the Atlantic Ocean and is rich in decaying organic matter. Imaged in Nomarski DIC using Olympus BH2 under SPlan 40x objective plus variable phone cropping on Samsung Galaxy S9+.
A diplomonad dance party. Today's slide from the northernmost saprobic edge benthos of spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve got richer and richer in diplomonad flagellates the longer the slide sat on the stage.
Thanks to Ivan Čepička for identifying this observation. This is the most numerous diplomonad species I observed in a slide that was teeming with diplomonads. The cells measured 10 μm. They were strongly flattened and oval in shape, the rear end was usually wider than
front. Cells moved relatively slowly compared to Trepomonas agilis and T. steinii and changed direction in a calm and fluid manner. The cells rotated vigorously as they moved. These findings were similar to (1).
"Diplomonad flagellates include the genera Trepomonas and Hexamita. Most genera of diplomonads are parasites, and the few genera that are free-living are usually found in organically enriched (and usually anaerobic) sites. The cells are bilaterally symmetrical along their longitudinal axis. There are two anterior nuclei, and associated with each are four flagella which arise at the head of a groove in the body surface. The genera may be distinguished by the relative lengths of the flagella and by the flexibility of the bodies. In both genera, one flagellum of both quartets extends laterally from the head of the groove. The remainder lie within the groove, with those of the more pliable Trepomonas not extending beyond the posterior margin of the cell, as do those of Hexamita . These organisms may feed either by eating bacteria or by pinocytosis" (2).
-
Eva Rmoutilová Thesis: Diversity of free-living diplomonads. Trainer: doc. RNDr. Ivan Čepička, Ph.D.Prague, 2015 Charles University in Prague
the Faculty of Natural Science.
Fotos / Sonidos
Qué
Género HexamitaObserv.
peptolabDescripción
Hexamita species (thanks to Alastair Simpson and Ivan Čepička for the ID) A diplomonad flagellate measuring 9 um from the intertidal benthos of marine estuary Acabonac Harbor at Louse Point imaged in Nomarski DIC on Olympus BH2 under SPlan 40x objective plus variable phone cropping on Samsung Galaxy S9+.
It is difficult to pin down the species. In trying to do that, I accessed a number of texts from the late 1800's and early 1900's. In crudely trying to match pictures, strangely the closest match I could find to my observation was the genus Urophagus, which at that time used to be classified with the Hexamitidae but now is classified: Urophagus G.A.Klebs Heterokonta (Infrakingdom) Ochrophyta (Phylum) Ochrophyta incertae sedis (Subphylum) Urophagus (Genus). Species Urophagus caudatus Skuja, 1939 Species Urophagus rostratus (Stein) Klebs (uncertain). I include illustrations from those texts for interest and completeness but of course I go with the opinions of my expert friends.
Genus Hexamita Dujardin, 1838. Parent Hexamitidae Kent, 1880. Marine species: Hexamita capelani Lavier, 1936; Hexamita inflata Dujardin, 1841 ; Hexamita salmonis (Moore, 1923) Wenyon, 1926. Also two species transferred to Spitronucleus: Hexamita phycidis Lavier, 1936 accepted as Spironucleus phycidis (Lavier, 1936) (synonym) and Hexamita salpae Lavier, 1936 accepted as Spironucleus salpae (Lavier, 1936) (synonym).
"The diplomonads (suborder Diplomonadida, family Hexamitidae) are a group of aerotolerant anaerobic flagellates, which possess a double set of cellular organelles. Amongst the diplomonad genera are Hexamita, Giardia and Spironucleus]. Species of Hexamita are mostly free-living organisms that reside in anaerobic water sediments whereas the other taxa are almost exclusively parasites, which commonly inhabit the intestinal tract of mammals, birds, reptiles, amphibians and fish. Diplomonads are members of the super-group of protista defined by an asymmetric feeding groove excavated from one side and hence termed the “Excavata”. They are thus flagellated eukaryotes that are taxonomically related to the Parabasalids and Euglenozoa. Discussion still revolves about their ‘primitive status’, i.e., whether they are early-branching eukaryotes, or crown taxa. They are characterized by their possession of two haploid nuclei, each associated with four flagella. In Spironucleus spp. the paired nuclei taper anteriorly and are wrapped around each other at their apices, forming an S-shape when viewed in transverse section of the anterior end of the cell. In other diplomonads the exact shape and location of nuclei are diagnostic for genus" (1).
- Comparative biochemistry of Giardia, Hexamita and Spironucleus: Enigmatic diplomonads. David Lloyd, Catrin F. Williams. Molecular and Biochemical Parasitology. Volume 197, Issues 1–2, October 2014, Pages 43-49
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken on 10/14/2023 using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Pinnularia formica; 120µ L x 18µ. Third encounter with this taxon. There presence appears specific to just 1 of the ~4 local waterbodies I've been monitoring, https://www.inaturalist.org/observations?place_id=any&taxon_id=471765&user_id=mnold1&verifiable=any
Reference: https://diatoms.org/species/pinnularia_formica.
Fotos / Sonidos
Observ.
lamawebberDescripción
Genus: Gomphonemopsis Medlin, 1986
Species:
Gomphonemopsis exigua Medlin 1986: 207. (Basionym: Gomphonema exigua (Kutz)). Valves linear to narrowly linear lanceolate, 9 to 34 pm long and 2 to 6 pm wide (Figs 1-2). Striae 16-30 in 10 pm, slightly radiate to parallel at the apices. Narrow axial area, no central area (Medlin and Round 1986). Morphology of the MHMPP specimens fit the material from Oregon, USA, except the central area is very slightly open. [Gomphonemopsis cf. exigua spp-Zm MHMPP-Box 0_stb 21-Mar 7-2021 CR-TM4000 (Aug 26-2021)_027(x1.5k)_3.tif at 28.4 µm long would not fit the length characteristics of any other Gomphonemopsis species except for Gomphonemopsis exigua.
Found on Z. marina. Collected at Montague Harbour Marine Provincial park (MHMPP). on March 7, 2021.
This is a first report of Gomphonemopsis exigua in the Salish Sea.
Taxonomic classification:
Phylum Bacillariophyta
Subphylum Bacillariophytina
Class Bacillariophyceae
Subclass Bacillariophycidae
Order Cymbellales
Family Rhoicospheniaceae (Guiry and Guiry 2021)
Type species, synonym(s), etc.: Gomphonemopsis exigua (Kützing) Medlin is the type species (holotype) of this genus (Guiry and Guiry 2021). Homotypic Synonym(s) Gomphonema exiguum Kützing 1844 and Gomphoneis exigua (Kützing) Medlin 2008 (Guiry and Guiry 2021)
An excellent summary of genus characterisic is found at: https://www.algaebase.org/search/genus/detail/?genus_id=44116
Most Gomphonemoid species produce stalks of mucopolysaccarides that are secreted through an apical pore field. Gomphonema, a freshwater genus has a distinctive basal (foot pole) apical pore field, whereas Gomphonemopsis has a row of pores. Gomphonema can be washed into coastal waters, but the MHMPP specimens are on intact stalks and likely too numerous on the Z. marina leaf sections to be a stray event.
Gomphonemopsis is a small genus in number of species and small in size. It is an epiphytic diatom of marine habitats. Widespread, but not found in large numbers. None of the Gomphonemoids were reported by Shim (1976) for the Straight of Georgia, and Tynii (1986) as forms of Nitzschia (i.e. N. hungarica). Gomphonemopsis (as Gomphonema exiguous Kutz = Gomphonemopsis exigua was recorded by Jacobs and Noten (1980) on Z. marina. AlgaeBase reports nine currently accepted species of Gomphonemopsis (Guiry and Guiry 2021).
Methods:
Collected by brushing or scrapping leaf sections of Z. marina from MHMPP, August 3, 2020, October 16, 2020, March 7, 2021 and July 2021. Cells were cleaned with concentrated hydrogen peroxide or nitric acid at 100 C for 3-5 hours to remove organics, then rinsed multiple times in ddH20 to a neutral pH. Mounted on SEM stubs or in Naphrax on coverslips for light microscopy. Imaging with a Nikon TE300 and Tuscen DigiRetna16 MP camera or Nikon E800.
Live specimens were imaged with either a Nikon TE300 or Nikon E800 with either bright-field or DIC. In-situ, environmentally prepared samples were made using minimal contact of 8-10 mm leaf section, soaked in ddH2O to remove salts and dried through an EtOH series (50%-100%) and finished off with 100% Hexamethyldisilane HMDS (Hazrin-Chong and Manefield 2012). Mounted on carbon stickies onto aluminum SEM stubs and imaged with either the Hitachi s4800 or TM4000 at AMF, at University of Victoria, B.C. My thanks to Siobhan Schenk and Laura Parfrey in the Parfrey Lab at UBC for molecular data from the eelgrass and collaboration with IMERSS. Also thanks go to Elaine Humphrey of the AMF, UVIC, imaging by Arjan van Asselt, Elaine Humphrey, Melanie Quenneville, Ron Read, Cole Ramsay. Additional imaging, taxonomy and identifications by M. Webber.
References:
Al-Handal, A., Thomas, E. W., Torstensson, A., Jahn, R. & Wulff, A. (2018). Gomphonemopsis ligowskii, a new diatom (Bacillariophyceae) from the marine Antarctic and a comparison to other Gomphonemopsis. Diatom Research 33: 97–103.DOI: 10.1080/0269249X.2018.1428916
Manal Al-Kandari, Dr. Faiza Y. Al-Yamani, Kholood Al-Rifaie (2009). Marine Phytoplankton Atlas of Kuwait’s Waters. Kuwait Institute for Scientific Research
Ehrenberg, C.G. (1832). Über die Entwickelung und Lebensdauer der Infusionsthiere; nebst ferneren Beiträgen zu einer Vergleichung ihrer organischen Systeme. Abhandlungen der Königlichen Akademie Wissenschaften zu Berlin, Physikalische Klasse 1831: 1-154, pls I-IV.
Frenguelli (1942). Diatomeas del Neuquen (Patagonia). INSTITUTO DEL MUSEO DI: LA UNIVENSIDAD NACIONAL DE LA PLATA, REVISTA DEL MUSEO DE LA PLATA. Tomo V. Botanica no. 20. (pp177-178. Lamina VIII fig. 12 (Tryblionella hungarica))
Guiry, M.D. in Guiry, M.D. & Guiry, G.M. 2021. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on July 6, 2021.
Hendey, N.I. (1964). An introductory account of the smaller algae of British coastal waters. Part V. Bacillariophyceae (diatoms). Her Majesty’s Stationary Office, London. p. 280 (as Nitzschia acuminata), plate 39, fig. 8 & 10.
Jacobs, R.P.W.M., Noten, T.M.P.A., 1980. The annual pattern of the diatoms in the epiphyton of eelgrass (Zostera marina L.) at Roscoff, France. Aquat. Bot. 8, 355-370.
Kaleli, M.A., Kulikovskiy, M.S., & Solak, C.N. (2017). Some New Records for Marine Diatom Flora of Turkey From Akliman, Sinop (Black Sea). Turkish Journal of Fisheries and Aquatic Sciences, 17, 1387-1395. pp. 1389-1390, Fig. 2: 15-18: Gomphonemopsis obscura (Krasske). http://doi.org/10.4194/1303-2712-v17_6_32
Kociolek, J.P., Spaulding, S. A., and Lowe, R. L. (2015). The Raphid Diatoms. Chapter 16, pp. 757-758. In: J. Wehr, R. Sheath & J.P. Kociolek [Eds], Freshwater Algae of North America. 2nd Edition. Academic Press, NY.
Krzywda, M., Gastineau, R., Bąk, M., Dąbek, P., Górecka, E., Chengxu, Z., Lange-Bertalot, H., Li, C. L., and Witkowski, A. (2019). Morphology and molecular phylogeny of Gomphonemopsis sieminskae sp. nov. isolated from brackish waters of the East China Sea coast. Plant and Fungal Systematics, 64(1), pp.17-24. https://doi.org/10.2478/pfs-2019-0003
Lobban, C.S., M. Schefter, R.W. Jordan, Y. Arai, A. Sasaki, E.C. Theriot, M. Ashworth, E.C. Ruck & C. Pennesi. 2012. Coral-reef diatoms (Bacillariophyta) from Guam: new records and preliminary checklist, with emphasis on epiphytic species from farmer-fish territories. Micronesica. 43(2): 237-479.
López-Fuerte, F.O., Siqueiros-Beltrones, D.A. & Navarro, N. (2010) Benthic diatoms associated with mangrove environments in the northwest region of Mexico. CONABIO–UABCS–IPN, 206 pp. p. 64: Gomphonemopsis littoralis (Hendey) Medlin. p. 161, Plate 28 fig. 20.
JOON SANG PARK, CHRISTOPHER S. LOBBAN & KYUN-WOO LEE Diatoms associated with seaweeds from Moen Island in Chuuk Lagoon,
Micronesia, Phytotaxa 351 (2): 101–140. https://doi.org/10.11646/phytotaxa.351.2.1 Fig. 33. Gomphonemopsis littoralis [24. Gomphonemopsis littoralis (Hendey) Medlin Fig. 33 References: Medlin & Round (1986), p. 210, figs 12–15, 52–54; Lobban et al. (2012), p. 264, pl. 22, figs 1–3. Samples: Moen1_Padina Dimensions: 24.3 μm long, 1.8 μm wide; 25 striae in 10 μm.]
Mather et al. (2010). A Checklist of Diatom Species Reported (and Presumed Native) from Canadian Coastal Waters. Fisheries and Oceans Canada (https://publications.gc.ca/collections/collection_2010/mpo-dfo/Fs97-6-2881-eng.pdf).
Medlin, L.K. & Round, F.E. (1986). Taxonomic studies of marine gomphonemoid diatoms. Diatom Research 1(2): 205-225, 100 figs, 1 table.
Pienitz, R., Fedje, D. and Poulin, M. (2003) Marine and Non-Marine Diatoms from the Haida Gwaii Archipelago and Surrounding Coasts, Northeastern Pacific, Canada In Bibliotheca Diatomologica (H. Lange-Bertalot and P. Kociolek, eds.), Band 48, J. Cramer, Stuttgart, 146 pp.
Plinski, M. & Witkowski, A. (2020). Diatoms from the Gulf of Gdansk and surrounding waters (the southern Baltic Sea). pp. [1]-442, incl. 31 SEM pls, 16 photo pls. Gdansk: Gdansk University Press.
Round, F.E., Crawford, R.M. and Mann, D.G. (1990), The Diatoms, Biology & Morphology of the Genera, pp. 614-615. Cambridge University Press, Cambridge, UK.
Silva, J. G., Cardoso, L. d. S., and Torgan, L.C. (2010). Salt Marsh Diatoms (Bacillariophyceae) in South Brazil. Acta bot. bras. 24(4): 935-947.
Siqueiros Beltrones, D.A., Mart nez, Y.J. & A. Aldana-Moreno (2019). Exploratory floristics of epiphytic diatoms from Revillagigedo Islands (Mexico). Cymbella 5(1): 98-123. http://cymbella.mx
Totti, C., Cucchiari, E., De Stefano, M., Pennesi, C., Romagnoli, T., & Bavestrello, G. (2007). Seasonal variations of epilithic diatoms on different hard substrates, in the northern Adriatic Sea. Journal of the Marine Biological Association of the United Kingdom, 87(3), 649-658. doi:10.1017/S0025315407054665
Tynni, R. 1986. Observations of diatoms on the coast of the state of Washington. Geological Survey of Finland, Report of Investigation 75.
Witkowski A, Lange-Bertalot H, Metzeltin D (2000) Diatom flora of marine coasts I In: Lange-Bertalot H (ed) Iconographia Diatomologica. Annotated Diatom Micrographs. Diversity-Taxonomy-Identification. Vol. 7 A.R.G. Gantner Verlag K.G., Ruggell, pp. 1–925.
Fotos / Sonidos
Qué
Orden SessilidaObserv.
mhinczDescripción
Telotroch (free-swimming) phase of a peritrich ciliate. Maybe Vorticellidae? Collected from freshwater stream, 400x magnification.
Fotos / Sonidos
Qué
Ciliado (Coleps hirtus)Observ.
davidfbirdDescripción
Three posterior spines; . 20 longitudinal rows of plates; body fairly plump; length 50 microns. (Noland, Lowell E. 1925. A Review of the Genus Coleps with Descriptions of Two New Species. Trans. Am. Microsc. Soc. 44(1): 3-13.)
Noland calls them 'micra', an interesting usage which had apparently faded by about 1950.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 100x (4), 400x (1-3)
- A pond-side water sample (retentate) was taken using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Heliozoan filled with zoochlorella. Note that shorter spine-scales are bifurcated, which is characteristic of A. turfacea. For information and reference images, see https://arcella.nl/acanthocystis-turfacea/.
Fotos / Sonidos
Qué
Arcella costataObserv.
mnold1Descripción
Mag. 400x
- A pond-side water sample (retentate) was taken using a 10µ dip net to enrich for microorganisms. Air temp 58F.
Flat apex. Widest measure, 85µ; collared, round aperture, 18µ. As seen and described here https://arcella.nl/arcella-costata/.
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A water sample was taken on 09/03/2023 from pond-edge using a 10µ dip net to enrich for microbes. Air temp. 80°F.
Amazingly pink ciliate. For a reference see the observation by Bruce Taylor, https://www.youtube.com/watch?v=E0bDRJTc50c.
Qué
Orden SessilidaObserv.
woodilljDescripción
- sampled from puddle just after rain
- objective (x10): 200x
Qué
Dictyocha fibulaObserv.
woodilljDescripción
- from marine sample (sand and water), collected at shoreline
- objective(s) (x 10): 1000x
- ID: ELPB-LGT40
Fotos / Sonidos
Observ.
woodilljDescripción
- from marine sample (sand and water), collected at shoreline
- objective(s) (x 10): 1000x
- approximate dimensions: see image 2
- ID: ELPB-LGT40
Fotos / Sonidos
Qué
Género SpirogyraObserv.
mnold1Descripción
Mag. 100x (1), 400x (2, 3)
- A pond-side water sample was taken on 07/31/2023 using a 10µ dip net to enrich for microbes. Air temp. 75°F.
A brobdingnagian-sized Spirogyra in decline!? In the left cell, the ribbon-like chloroplasts (regularly dotted with small round pyrenoids) have "detached" from the cell wall; actually it appears that the ribbons (all ~10µ wide) are enclosed in a membranous bag... perhaps they are attached to this membrane. In the right cell, the chloroplasts are coiled and the surrounding membrane is further contracted compared to the left cell. Also interesting is the size of this 2 cell segment of Spirogyra; at ~150µ wide and ~300µ long, these Spirogyra cells are huge! Yes? ...or Not Spirogyra?... (I estimate at least 10 ribbon chloroplasts per cell. The cell size and # of chloroplasts suggest S. crassa as a possible ID, http://protist.i.hosei.ac.jp/pdb/images/chlorophyta/Spirogyra/group_A/sp_08.html.)
Fotos / Sonidos
Observ.
mnold1Descripción
Mag. 400x
- A pond-side water sample was taken on 07/31/2023 using a 10µ dip net to enrich for microbes. Air temp. 75°F.
As seen here https://www.algaebase.org/search/images/detail/?img_id=18232 and https://www.desmids.nl/maand/english/closterium_acutum.html.
Apex-Apex = 80µ
W = 4µ
Fotos / Sonidos
Qué
Género HalteriaObserv.
shanesmicroscopeDescripción
Continued observation of Bird Bath S1. S1 had dried completely 20210628 during a wave of abnormally hot weather. Rain came late in the evening of 20210630. It has remained hydrated since through the occasional rain.
Vorticella and halteria present in small numbers. No other ciliates observed. Numerous small flagellates. Tetradesmus algae plentiful.
Another stuck/damaged Halteria demonstrating that the "jumping" movement is indeed more of a fast swimming action.