Qué
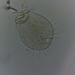
Colibrí Garganta Rubí (Archilochus colubris)Observ.
rickcechDescripción
Ruby-throated Hummingbird
Qué
Piranga Escarlata (Piranga olivacea)Observ.
altspenDescripción
Bird banding breakfast event. First male Scarlet Tanager at the nets!
Fotos / Sonidos
Observ.
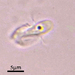
peptolabDescripción
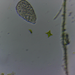
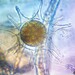
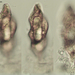
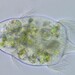
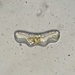
Spathidiopsis socialis Fabre-Domergue, 1889 from the intertidal benthos of marine estuary channel between Three Mile Harbor and Gardiner's Bay. Imaged in Nomarsdki DIC on Olympus BH2S using SPlanapo 40 0.95 and SPlan 100 1.25 oil objective with oiled condenser, plus variable phone camera cropping on Samsung Galaxy S9+.
The cells measure 70 um in length. I have shown this species before, but this is the first time I imaged it under oil immersion and found individuals with few food vacuoles which in the prior observations obscured the macronucleus. Here it is clearly seen as an elongated sausage shape making certain the diagnosis of S. socialis. The cell body is ovoid to reniform and somewhat dorsoventrally flattened and shows a shallow "inpocketing" depression 1/3 to 1/2 of the way down from the apex. Again, this is the first time I was able to demonstrate that the inpocketing contains many rod-shaped toxicysts. The cell surface is sculptured into a system of longitudinally spiraling ridges and furrows. The cytostome is apical and slit like. There is a delicate cytopharyngeal basket of nematodemal rods. There is a prominent row ("pseudomembrane") of stiff toxicyst-bearing palps running from the cytostome and terminating at the inpocketing. These palps accompany and overshadow a brosse of the same length. There is a large posterior contractile vacuole. The animals tend to cluster together in the same area. Based on the size, swimming habit, prominence of the row of palps accompanying the brosse, and macronuclear shape, these features are consistent with Spathidiopsis socialis. Kahl, 1926 described this inpocketing or Gru¨bchen on its lateral surface and a “Pseudomembrane” (i.e. the row of toxicyst bearing palps adjacent to the brosse).
Lipscomb and Riordan have extensively studied and clarified the genus Spathidiopsis and its relationship with its sister taxon Placus and their confusing and convoluted taxonomy and nomenclature (1,2). "SPATHIDIOPSIS Fabre-Domergue, 1889 is a reniform, prostomatid ciliate found swimming over surfaces in marine, brackish, and freshwater habitats in search of other protists as prey. The members of this genus and its sister-taxon, Placus, are the only two genera within the family Placidae (Small and Lynn 1985). The family has been placed in the class Prostomatea (Schewiakoff 1896) and order Prorodontida (Corliss 1974) because its members have somatic monokinetids with a radial transverse ribbon, a straight nonoverlapping postciliary ribbon, and anteriorly directed nonoverlapping kinetodesmal fibril, an apical cytostome lacking complex oral cilia, a brosse, and toxicysts (Corliss 1979; Grain et al. 1978; Lynn 2008; Lynn and Small 2002; Small and Lynn 1985)" (1). Placus lacks the "pseudomembrane" of toxicyst-bearing palps seen in Spathidiopsis.
Liposcomb and Riordan redescribed and revised the family Placidae and the genus Spathidiopsis: "Genus Spathidiopsis Fabre-Domergue, 1889 Synonyms. Thoracophrya Kahl, 1926 (in part); Thoracophrya Kahl, 1928 (in part); Toracophrya Klein, 1928; Placus Kahl, 1930 (in part); Placus Curds, 1982.
Species. Spathidiopsis socialis Fabre-Domergue, 1889 (type by monotypy); Spathidiopsis buddenbrocki Sauerbrey, 1928; Spathidiopsis luciae Kahl, 1926; Spathidiopsis eforianus Tucolesco, 1962. Diagnosis. Reniform to ellipsoid placid ciliates with an inpocketing and “pseudomembrane” (i.e. row of toxicyst-bearing palps adjacent to the cytostome and brosse). The inpocketing contains many rod-shaped toxicysts. The brosse consists of a row of paired kinetosomes and a row of single kinetosomes that begins adjacent to the cytostome and continues down the ventral side one-third to one-half the length of the cell to terminate in the inpocketing. The cytostome is slit-like and surrounded by paired oral kinetosomes that lack cilia". (1).
These authors describe a wide size range for S. socialis: Spathidiopsis socialis Fabre-Domergue, 1889
Synonyms. Thoracophrya marina Kahl, 1927; Thoracophrya lucia var. livida Wang and Nie, 1932; Placus socialis Kahl, 1930; Placus salinus Dietz, 1964; Placus salinus Borror, 1972; Placus striatus Grain et al., 1978; Spathidiopsis striatus Small and Lynn, 1985; Spathidiopsis salinus Carey, 1992; Placus dogieli Burkovsky, 1970; Spathidiopsis dogieli Carey, 1992; Placus marina Foissner et al., 1994; Placus salinus Xu et al., 2005.
Description. Ovoid, 32–130 um long by 24–82 um wide. Laterally flattened. Cytostome slit-like. Spiraling kineties, 30-32, usually 31. Tends to aggregate into groups, hence the species name, socialis.
- The Ultrastructure of Placus striatus and a Revision of the Family Placidae (Ciliophora) DIANA L. LIPSCOMB and GAVIN P. RIORDAN. J. Eukaryot. Microbiol., 59(4), 2012 pp. 407–422
- A Molecular and Ultrastructural Description of Spathidiopsis buddenbrocki and the Phylogenetic Position of the Family Placidae (Ciliophora). DIANA L. LIPSCOMB, BRUNELLA M. BOWDITCH and GAVIN P. RIORDAN. J. Eukaryot. Microbiol., 59(1), 2012 pp. 67–79
Fotos / Sonidos
Observ.
closterium_mysteriumDescripción
Video: https://youtu.be/FTEfuyBeFbY
Sampling location: A water sample was taken from the bank of the Vuoksi River.
Date and time of collection: 22 Jul 2023 at 12 PM
Date and time of observation: 23 Jul 2023 at 9 PM
The sample was stored at room temperature in a plastic container.
All images and videos are of the same organism.
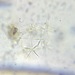
Taphrocampa selenura Gosse, 1887
Diagnosis:
• caterpillar-like body
• body length ~270 µm
• trophi length ~40 µm
• the mastax is virgate with strongly asymmetric trophi
• toes length ~30 µm
• foot rudimentary, toes long, slender, tapering and decurved; they are wide apart at the base and form almost a semicircle when viewed dorsally or ventrally; their length is about one-ninth of total length
• a concertina-like integument
• dorsal epidermis is sticky with transverse folds and it is abundantly covered with adherent foreign particles
• auricles retractable
• retrocerebral organ with attached eyespot
• two small symmetrically placed dorsal antennas on the head
• at least one small antenna at the posterior end, above the toes
• it appears that there are lateral antennas (?)
References:
Rotifer fauna of the USSR (Rotatoria). Subclass Eurotatoria (Orders Ploimida, Monimotrochida, Paedotrochida) (221, Fig. 99) by L.A. Kutikova (1970)
Observations by Michael Plewka https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/E-TL/Genus/Taphrocampa.html
Observation by Dr. Martin Kreutz https://realmicrolife.com/taphrocampa-annulosa/
https://www.nies.go.jp/chiiki1/protoz/morpho/rotifera/r-taphro.htm#Taphrocampa%20selenura
http://www.rotifera.hausdernatur.at/HigherTaxonomy/Index
http://rotifera.hausdernatur.at/Rotifer_data/images/addscan/_full-size/Taphrocampa%20selenura%20Gosse,%201887%20[Harring%20et%20Myers,%201924].jpg
Fotos / Sonidos
Observ.
polarblairxDescripción
Growing on a clay slope behind the Mabel Smith Douglass Library, with antheridial cups, few sporophytes
Fotos / Sonidos
Observ.
peptolabDescripción
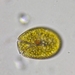
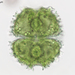
Caenomorpha medusula PERTY, 1852 from the superficial river edge benthos of the freshwater segment of the estuarine Peconic River. Imaged in Nomarsdki DIC on Olympus BH2S using Splanapo 40 0.95 and Splan 100 1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+.
This metopid ciliate is a fast swimmer and often difficult to track, but also holds on to the substrate thigmotactically with the dorsally located long cirri. It is an inhabitant of saprobic anoxic decaying benthic organic debris. My population averages 100 um in total length including the single long caudal spine. There are three macronuclear nodules with two sometimes incompletely separated and a single micronucleus. There is an anterior refractile granular agggregate. The cytoplasm contains diverse rod-shaped bacterial symbionts. The contractile vacuole was rather large in diastole in some individuals. All of these features are in agreement with the most recent redescription by Li et al 2017 (1).
Li et al 2017 provide the most recent and detailed redescription of the species. In describing the most important characters, they write: "Although this species has been studied for more than 150 years, species identification remains problematic because many descriptions are based only on live materials without redescription of the ciliature. Furthermore, some features, e.g. body shape, appear to vary among individuals in different environments, but, nonetheless, have traditionally been used as key characters in the taxonomy of Caenomorpha. The original description of Caenomorpha medusula by Perty (1852) was rather superficial and failed to note some important features (e.g. the numbers of adoral membranelles, bell kineties and spines, etc.), which renders the identification of this organism difficult. However, according to the original and subsequent investigations, this species can be recognized by a combination of the following characters: (i) multiple macronuclear nodules; (ii) two unequal-length bell kineties; (iii) one conspicuous posterior spine" (1).
"Description: Body medusoid, covered with a transparent rigid pellicle, 112–125 × 50–65 um in vivo with a ratio of length to width about 2:1. Posterior spine slender, about 45–55 um long in vivo; ratio of spine length to body length about 0.4. Plump rod-shaped epibiotic bacteria often found in US population but not found in China population. Cytoplasm clear and colourless, with some dark globules (1–2 m across) and an aggregate of transparent granules in anterior body part, rod-like bacteria exist in cytoplasm in China population, very likely endosymbionts. Edges of preoral bell never adjoin closely to posterior body; peristome narrow, deep funnel-shaped. Cytostome near base of spine; undulating membrane recognizable after staining, length of membrane about 50 m long. Contractile vacuole located near base of spine, about 15–20 m in diameter, pulsates at intervals of 3–5 min. Three (41 of 60 cells [Chinese population], 36 of 45 cells [US population]), four (19 of 60 cells [Chinese population], 8 of 45 cells [US population]) or five (1 of 45 cells [US population]) macronuclear nodules, usually ovoid or ellipsoidal, arranged in line, located in center of cell, sometimes incompletely separated; one micronucleus, ellipsoidal, near macronuclear nodules. Movement leisurely, spiraling while rotating around the long axis of the body" (1).
"Two strongly thigmotactic bell kineties about 68 um and 35 um long, respectively, located in anterior part of dorsal side of cell, consist of about 94 and 56 cirri (Chinese population), and 108 and 59 cirri (US population) respectively (n = 21); cirri in each kinety arranged in indistinct zig-zag pattern. Perizonal stripe beginning near anterior end of cell, about 6.4 um wide at middle part, composed of 114–180 kineties (114–169 in China population, 123–180 in US population), spiraling 450 degrees around axis; each kinety inclined about 60 degrees to edge of shield; longest kinety (at middle of stripe) composed of about 15 pairs of kinetosomes in both populations, whereas ones near oral region with only two pairs of kinetosomes. Adoral zone composed of 41-67 membranelles (Chinese population), each with three or four rows of kinetosomes, spiraling 360 degrees around body axis from near the distal perizonal stripe, terminates near cytostome. Undulating membrane on undersurface of preoral bell (i.e. roof of peristomial region), about 50 m long. Cilia on base of spine, invariably arranged in two short kineties, each 10–15 m long, composed of about 25 kinetosomes each. The two spine kineties inclined about 20 degrees to each other, converge posteriorly" (1).
Jankowski provided an older but elegant description: "Body medusoid, covered with a clear transparent rigid pellicle that looks like an armour; body length, without a spine, is 70-90 x 65-70 ul, spine 30-35 um long. The shield bears no ciliary meridians except for those of a perizonal ciliary stripe. Instead. it bears two groups of long thick flexible cirri, with 8-10 cirri in each group: they are perfectly seen from the left side. These cirri are highly thigmotactic- one can frequently observe a prolonged adhesion of the animals to sapropelic particles by the aid of these cirri, while both perizonal cilia and adoral membranelles continue their activity. The edges of the shield never adjoin closely to the body surface; instead, a narrow deep funnel may be observed between them. The perizonal ciliary stripe occupy the margin of the shield; it is composed of 5 ciliary rows not separated into two groups, unlike that of Metopidae (where it includes 2 upper and 3 lower kinetics). The PCS of Caenomorpha looks like a wide densely ciliated field composed by a number of short oblique ciliary rows with 5 kinetosomes each. It serves for both feeding and movement in this genus. The synchronous beating of the perizonal cilia and adoral membranelles produces a rotation of the swimming animal and, in addition, creates the intense water current along the buccal groove. driving the food-particles into the intrastomium. The cytostome in C. medusula occupies a typical for all the caenomorphids posterior position with a thin tubular cytopharynx raising right up into the anterior body part, where colourless food vacuoles are concentrated" (2).
- Description of two species of caenomorphid ciliates (Ciliophora, Armophorea): Morphology and molecular phylogeny. Song Li, William A. Bourland, Saleh A. Al-Farraj, Lifang Li and Xiaozhong Hu. European Journal of Protistology 61 (2017) 29–40.
- Morphology and Evolution of Ciliophora. III. Diagnoses and Phylogenesis of 53 Sapropelebionts, Mainly of the order Heterotrichida. ANATOL W. JANKOWSKI. Arch. Protistenk. Bd. 107, S. 185-294 (1964)
Fotos / Sonidos
Observ.
dgborinDescripción
5 specimens, but I'm not really sure they are the same species.
Fotos / Sonidos
Observ.
peptolabDescripción
Spirostomum ambiguum Ehrenberg, 1834. from the superficial river edge benthos of the freshwater segment of the estuarine Peconic River. Imaged in Nomarsdki DIC on Olympus BH2S using Splanapo 20 0.70 and SPlanapo 40 0.95 objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cell measures 960 um in length. The peristome is 2/3 the length of the body with the cytostome at the posterior 2/3 location. There is a large terminal contractive vacuole with a long collecting canal reaching almost the anterior end of the cell. The macronucleus is moniliform forming a chain of connected elongate spindle shaped macronuclear nodules beginning at the anterior 40% of the cell length and extending almost to the posterior end.
"Spirostomum ambiguum Ehrenberg, 1834. [syn: Trichoda ambiguum Müller, 1786; S. ambiguum var. major Roux, 1901] 900 m several mm long. Length: width ratio about 9-17. 15-25 kineties on each side; heterogeneous, numerous (4-5) CG rows per stripe. Peristome always longer than 1/2 of the body length, often reaching 2/3. CV much shorter than body length, rarely exceeding 1/10. The color depends on cytoplasmic granules. Moniliform MAC with 12-50 (avg. 15-25) nodules not exceeding 35 45 m in length when stained by Feulgen reaction. Numerous (up to 100) MICs 1-2 m long. Mono phyletic. Only found in freshwater. Reported in central and northern Europe, England, Russia, central Africa, USA, Jamaica, India and Japan. It sometimes harbors prokaryotic symbionts in the MAC. Spirostomum ambiguum is a well-defined, easily recognizable morphospecies whose monophyly is also strongly supported by molecular sequences" (1).
"Differential diagnosis
1) Size in vivo 1-000-4000 x 48-100 um, mostly 1200-2000 um. Visible with the naked eye as a white thread. Very flexible and contractile, shortens to approximately 390-430 x200-220 um. Contracted cells cigar-shaped.
2) Shape slender to moderately broad worm-shaped, more or less parallel-sided, 10-17 times longer than wide. Front end rounded, rear end truncated. Slightly flattened laterally. Ventral side in area of the mouth entrance slightly bulging.
3) Macronucleus moniliform or rosary-shaped, consists of 10-50, mostly 15-20 ellipsoids, about 18-53 x 12-24 um large nodes that form a long band that approaches the dorsal side. The number of nodes correlates positively with the age of the cell (REPAK & ISQUITH L974).
4) Contractile vacuole at the posterior end, with a long collecting duct extending forward along the dorsal side which sometimes shows ampullary extensions.
5) Close under the pellicle there are many spherical, spherical, yellowish granules arranged in elongated bands of 4-5 rows each which give the cell a yellowish to brownish color.
6) About 70-90 slightly spiraling rows of cilia, consisting of basal bodies arranged in pairs are constructed, but only the front one has a cilium.t
When the cells contract (startle reaction), the rows of eyelashes spiral around the body.
7) The adoral membrane zone extends from the anterior end to the posterior third (about 65-70 % of the body length) and turns to the right at the lower end. Parallel to the adoral membranelle zone a non-ciliated oral groove, which is bordered on the right by an undulating membrane.
8} Movement hatching, worm-like crawling and writhing. Fluidity with longitudinal rotation axis, with the front end describing a cone-shaped body of revolution. In the plankton falling floating with inclined longitudinal axis" (2).
"Spirostomum ambiguum is easy to recognize because of its size and shape, but the differentiation with S. minus causes considerable difficulties. There are many shape variations which occur, size and shape are not reliable distinguishing features. Usually S. minus is significantly slimmer and almost never reaches a length of 1 mm, while S. ambiguum is more compact, stockier and over 1 m long. Only the length of the adoral membrane zone in relation for body length (S. ambiguum: 65-70 %; S. minus: 35-50%) remains reasonably reliable distinguishing feature, but here too the differences do not seem to be too pronounced. Furthermore, the ciliates S. semivirescens have a similar size and shape (up to 2 mm, very slender, adoral membrane zone up to 50 % of body length, green through symbiontic algae) and Homalozoon vermiculare (up to 1.5 mm, 5-21 contractile vacuoles along the dorsal side, mouth small and only at the front end) as well as microturbellaria and nematodes. The characteristics 1,3 and 7 are particularly important for identification" (2).
- Focusing on Genera to Improve Species Identification: Revised Systematics of the Ciliate Spirostomum Vittorio Boscaroa,1, Daniela Carducci, Giovanna Barbieri, Marcus V.X. Senra, Ilaria Andreoli, Fabrizio Erra, Giulio Petroni, Franco Verni, and Sergei I. Fokin. Protist, Vol. 165, 527–541, August 2014
- FOISSNER W., BERGER H. & KOHMANN F. (1992): Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems - Band II: Peritrichia, Heterotrichida, Odontostomatida. – Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, 5/92: pp 317-25.
Fotos / Sonidos
Qué
Hongo Estrellita (Astraeus hygrometricus)Observ.
pratyekaDescripción
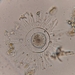
Seven revolute rays of the exoperidium are visible. Exoperidium interior is deeply fissured, maximum unbroken interior segment length near ray tip ≈5mm. Nearby angiosperm species providing potential host root systems include two Acacia species (at least one non-endemic), one non-endemic Grevillea and one Macrozamia communis. It is considered probable that an Acacia is the host. This could be verified after spore release is completed and the specimen is collected.
Fotos / Sonidos
Qué
Orden SessilidaObserv.
fmorales369Descripción
Sample taken from a bryophyte wash. Objective: 40x
Fotos / Sonidos
Qué
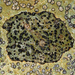
Diatomeas (Clase Bacillariophyceae)Observ.
ha300Lugar
121, Route Royale, Beau Bassin, Quatre Bornes, Port Louis District, Moka, Mauritius (Google, OSM)Qué
Pinos, Ocotes Y Piñones (Género Pinus)Observ.
christineyoungDescripción
Pine ovulate strobilus, 10x.
*Microscope slide of longitudinal section, stained
Fotos / Sonidos
Observ.
someplantDescripción
Magnification of all photos: 600×
Habitat: organic debris and decaying leaf matter from a freshwater pond. This one was observed four days after collection.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 600× is 100 µm.
Fotos / Sonidos
Qué
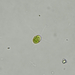
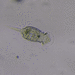
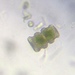
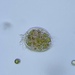
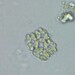
Género CarteriaObserv.
jordi_mvDescripción
Unicellular green algae with 4 flagella
400X
Eyepiece: WF10X DIN/18MM
Objective: S40/0.65 160/0.17
Sample of river water containing filamentous algae belonging to the genus Spirogyra.
Fotos / Sonidos
Qué
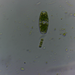
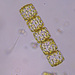
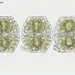
Género DiatomaObserv.
jordi_mvDescripción
400X
Eyepiece: WF10X DIN/18MM
Objective: S40/0.65 160/0.17
Sample of river water containing filamentous algae belonging to the genus Spirogyra.
Qué
Moscas de Ojos Saltones (Familia Diopsidae)Observ.
christineyoungDescripción
Pinned specimen; it was collected near Java, Indonesia.
Fotos / Sonidos
Observ.
trientalidDescripción
In thick gelatinous Spirotaenia endospira colony on wet boulder with mosses and lichens
Fotos / Sonidos
Observ.
peptolabDescripción
Stentor pyriformis Johnson 1893 from the superficial benthos of the river edge of the freshwater segment of estuarine river Peconic River. Imaged in Nomarski DIC on Olympus BH2S using SPlan 10 NA 0.30, SPlanapo 20 NA 0.70, SPlanapo 40 NA 0.95 and SPlan 100 NA1.25 oil objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cells were visible with the naked eye accumulating on the surface of the water at the side of the sample container. Cells measure from 650-800 um when fully extended. The cytoplasm is densely filled with green algal zoochlorella symbionts. There are also much smaller colorless cortical and cytoplasmic granules. The dense population of zoochorellae obscured the internal morphology of the stentors. Macronuclei could only be observed in markedly squashed cells by evaporation of water from under the coverglass. Most cells had two spherical macronuclei, occasionally three. The morphology of my population was identical to that described by Hoshina et al 2021, however their population was smaller than mine at 220-500 um and their macronuclear count also differed from my population which showed 2 MA: "The average number of macronuclei was 6.1 (range 4–10, n = 9) for freshly obtained samples, whereas four-year cultured cells contained only one or two" (1).
"S. pyriformis is a poorly described species, although S. pyriformis is clearly distinguishable from other Stentor species. The species was first described in 1893 and then appeared in a microbiota report in 1908. However, its next appearance was not until 1994, in the study on revision of the genus by Foissner and Wolfl. As described in the original literature, difficulties in the cultivation of this species may have hindered the research on this species. In Japan, S. pyriformis can be found only in highland highly oligotrophic moors, suggesting that intracellular symbiotic algae would help this species of Stentor survive in such a harsh environment. S. pyriformis was described by Johnson in 18936. This algae-bearing Stentor has separated spherical macronuclei without pigmentation, which certainly differentiates it from other Stentor species. While the most common algae-bearing Stentor, S. polymorphus assumes a slender trumpet shape (often shortened), S. pyriformis never resembles such a slender trumpet, but assumes a pear or short conical shape, even when it is swimming. Among algae-bearing Stentor spp., S. polymorphus and S. pyriformis only are considered colorless species, whereas colored species are S. amethystinus, S. fuliginosus, S. araucanus, and S. tartari. Therefore, S. pyriformis is a clearly discernible species; however, it remains underexplored" (1).
"Cells of S. pyriformis were broadly trumpet-shaped, usually 220–500 × 120–300 µm. This length–width ratio did not change significantly between the cells attached to something and swimming. The cells were colored green due to their endosymbiotic green algae that were distributed along the whole body. A large number of tiny transparent vesicles were present along the ciliary rows immediately under the cell surface. To see the contents, the crushed cells were observed. Symbiotic algae appeared to be typical Chlorella-like algae, but no dividing alga was observed. The algal cells appeared more vividly green when compared to those in P. bursaria, suggesting that they are richer in photosynthetic pigments. The symbiotic algae in S. pyriformis had the same size and morphology as those in P. bursaria. Macronuclei were, in general, large and spherical (ø 20–35 µm). The average number of macronuclei was 6.1 (range 4–10, n = 9) for freshly obtained samples, whereas four-year cultured cells contained only one or two. Micronuclei could not be identified" (1).
My population had two macronuclei rarely three in contrast to the populations of Hoshina et al which had an average of 6.1 macronuclei (range 4-10). Also, Hoshina et al 2021 (1) did not identify micronuclei in their multimodality study of S. pyriformis which included electron microscopy. However, the study of Walker 1908 (2) describes the presence of two macronuclei with multiple small micronuclei scattered within the macronucleus. I detected similar small nodules within the macronuclei to those depicted by Walker 1908 but I am uncertain if these are nucleoli or micronuclei. Hishina et al 2021 describe " a large number of transparent vesicles were present along the ciliary rows immediately under the cell surface (1) which I also found. In addition, Hoshina et al 2021 describe numerous starch granules in the cytoplasm which I believe are also present in my samples. These granules are more than twice the size of the subcortical vesicles.
- Characterization of a green Stentor with symbiotic algae growing in an extremely oligotrophic environment and storing large amounts of starch granules in its cytoplasm. Ryo Hoshina, Yuuji Tsukii, Terue Harumoto & Toshinobu Suzaki. Scientific Reports | (2021) 11:2865 | https://doi.org/10.1038/s41598-021-82416-9
- Observations on the Micro-Fauna of an Oregon Pond. Elda R. Walker. Transactions of the American Microscopical Society, Vol. 28, Twenty-Ninth Annual Meeting (Sep., 1908), pp. 75-84
Fotos / Sonidos
Observ.
peptolabDescripción
Spirostomum teres Claparède & Lachmann, 1859 from Pussy's Pond, a brackish offshoot of the estuary Acabonac Harbor. Imaged in Nomarski DIC on Olympus BH2 using SPlanapo 20 0.75, SPlanapo 40 0.95 objectives and Splan 100 1.25 oil objective plus variable phone camera cropping on Samsung Galaxy S9+. The cells measure from 480 up to 560 um in average length, have a compact ellipsoid macronuclear nodule, and are brownish in color and highly spirally contractile. A single row of well-developed oral membranelles defines the left side of the long, thin peristomial field, which runs parallel to the main body axis from the anterior end to the cytostome, which is located at 45% of the body length. One stripe of packed cortical granule rows (average 3) between each kinety pair; cortical granules are of different sizes. The contractile vacuole is single and posteriorly located, with a long collecting canal extending along the dorsal side often reaching the anterior end.
"Spirostomum is a genus of ciliated protists that belongs to the class Heterotrichea. It is known for being very contractile. Having been first identified by Christian Gottfried Ehrenberg in 1834, further research has identified eight additional true morphospecies. This bacterivore genus mainly lives in the sediment deposits at the bottom of various aquatic habitats, and members possess rquA genes that could be responsible for their ability to survive in these hypoxic and anoxic environments. They are identifiable by their relatively (to other ciliates) large tubular/flat vermiform bodies. Their life cycle consists of a growth stage, in which they mature, and asexual and sexual reproduction stages. Some species are model organisms for studies on human pathogenic bacteria, while others are sensitive and accurate bioindicators for toxic substances" (1).
"Spirostomum Ehrenberg, 1838 are conspicuous ciliates protists that are easily recognized by their large sizes (500-1000 µm) and elongate bodies, being easily confounded with small helminths. The name Spirostomum refers to the ability these ciliates have to contract in a spiral mode. This type of contraction is due to the presence of post-ciliary, sub-pellicular fibers that arise on the anterior end and spiral in a counterclockwise direction toward the posterior end of the body. Of the eight species that currently comprise Spirostomum, five (including S. minus) possess a moniliform macronucleus" (2). "There are currently eight accepted morphospecies in the genus: S. ambiguum, S. minus, S. teres, S. yagiui, S. dharwarensis, S. semivirescens, S. subtilis, S. caudatum" (1). Among all species of Spirostomum, only S. teres and S. caudatum have a compact macronucleus. S. caudatum is easily separated from S. caudatum by the latter's long thin tail (1). Another old species similar to S. teres is S. ephrussi but according to Repack and Isquith (1974), S. ephrussi is junior synonym of S. teres" (2).
"Spirostomum teres Cláparéde and Lachmann, 1858-1859. [syn: S. ephrussi Delphy, 1939] 150-650 um (avg. 250-450) um long. Length: width ratio about 5-16 (avg. 8-12). 5-15 (avg. 7-10) kineties on each side; usually homogeneous cortical granule rows, variable in number per stripe (2-4). Peristome from 1/3 to slightly more than 1/2 of the body length. Contractile vacuole usually less than 1/5 of the body length. Often brownish. Ellipsoid macronucleus (length: width ratio < 5) in the middle sector of the body, about 20-50 x 5-20 um when stained by Feulgen reaction. A few (1-3) micronuclei 1-2 m long. Molecular analyses suggest that this morphospecies include phylogenetically diverse lineages, some of which are more closely related to S. yagiui and S. dharwarensis; no reliable morphological autapomorphy has yet been detected for these lineages. Found in both fresh- and brackish-water environments. Reported in Europe, central Africa, Madagascar, USA, Brazil, Caspian Sea, India, China and Korea. It sometimes harbors cytoplasmic prokaryotic symbionts" (3).
"Differential diagnosis: 1) Size in vivo 150-600 x20-75 um, mostly 200-400 um, according to DRAGESCO (1960) brackish water forms even reach a length of 880 um. Very contractile; contractile cells more clearly spindle-shaped. 2) Shape moderately broadly cylindrical, somewhat expanded in the middle third, 10-15 times longer than wide. Anterior half distinct, posterior slightly narrowed. Front end slightly beaked, rear end broadly truncated. 3) Macronucleus ellipsoid, about 25-48 x 9-12 um in size, lies in the middle third. 1-2 lenticular, 2-4 um large micronuclei. 4) Contractile vacuole at the posterior end, with a long collecting duct extending forward along the dorsal side. 5) Close under the pellicle, very many spherical, arranged in elongated bands of 2-3 rows each lemon-yellow granules (oil immersion), which give the cell a yellowish tinge. 6) About 25-30 rows of cilia, few in stretched cells, strong in contracted individuals run spirally. 7) The adoral membranelle zone extends from the anterior end to about the middle of the body and bends to the right at the lower end. Oral groove bordered on the right by an undulating membrane. 8] Movement slipping, wriggling and crawling. Floats with rotation around the longitudinal axis" (4).
1, https://en.wikipedia.org/wiki/Spirostomum#cite_note-:3-1
-
Morphology and 18S rDNA gene sequence of Spirostomum minus and Spirostomum teres (Ciliophora: Heterotrichea) from Rio de Janeiro, Brazil. Noemi M. Fernandes & Inácio. D. da Silva Neto. ZOOLOGIA 30 (1): 72–79, February, 2013 http://dx.doi.org/10.1590/S1984-46702013000100009
Fotos / Sonidos
Observ.
peptolabDescripción
Trachelophyllum brachypharynx Levander, 1894
"Trachelophyllids prefer terrestrial and semiterrestrial habitats (Kahl 1930; Foissner 1984, 1994, 2005; Foissner et al. 2002), but some have also been found in freshwater (Foissner et al. 1995, 1999) and one species, Trachelophyllum brachypharynx Levander, 1894, was reported from saltwater (e.g., Levander 1894, 1901; Coats and Clamp 2009; Telesh et al. 2009)". I found a population of this species in brackish water from the estuarine pond Pussy's Pond, an offshoot of Accabonac Harbor in the Town of East Hampton, New York. Except for its somewhat smaller size, my observation conforms perfectly to the description of Jang et. al. Size is often variable intraspecies. The lepidosomes forming a mucilagenous cortical surface layer are notoriously difficult to visualize in vivo but you can clearly see the roughened character of the cortex in my photos.
"Size about 380 × 40 μm in vivo, slightly contractile. Shape very narrowly fusiform, with a slightly to distinctly narrowed neck, gradually merging into a broadened trunk. Two ellipsoidal macronuclear nodules usually connected by a fine strand, with two to three broadly ellipsoidal micronuclei close to or attached to macronuclear nodules. Contractile vacuole terminal and comparatively small. Extrusomes filiform, slightly curved and with pointed ends, 30 µm long in vivo. On average, 24 ciliary rows, two anteriorly differentiated into an isostichad dikinetidal dorsal brush: row 1 composed of 40 dikinetids on average, row 2 of 33 dikinetids on average. Lepidosomes hat-shaped and about 4 × 3.7 µm in vivo". "Oral bulge rather conspicuous because distinctly set off from body proper; pin-shaped in extended specimens, while conical in contracted cells; not covered by lepidosomes".
Another marine Trachelophyllum species is Trachelophyllum apiculatum (Perty, 1852) Claparède & Lachmann, 1859. However this species is more flask shaped and has four macronuclear nodules.
Morphology, Ciliary Pattern and Molecular Phylogeny of Trachelophyllum brachypharynx Levander, 1894 (Litostomatea, Haptoria, Spathidiida) Seok Won JANG, Peter Vďačný, Shahed Uddin Ahmed Shazib and Mann Kyoon Shin. Acta Protozool. (2015) 54: 123–135
Fotos / Sonidos
Observ.
pierrelfrDescripción
Collected on April 10, 2021. Length 150µ->250. Last photo is gif showing flatness.
Fotos / Sonidos
Observ.
davidfbirdDescripción
From a meltwater puddle on the floor of an abandoned limestone quarry. Length 110 to 125 µm. Zoochlorellae. I put a longer video here: https://www.facebook.com/100070304167055/videos/267405716339712.
Fotos / Sonidos
Qué
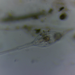
Orden CraspedidaObserv.
someplantDescripción
Magnification of photos: 400×, 400×, 400×, 400×, 400×, 600×, 600×
Habitat: filamentous green algae (mostly Mougeotia and Spirogyra) and some organic debris collected from a freshwater pond.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 400× is 150 µm.
Fotos / Sonidos
Observ.
someplantDescripción
Magnification of photos: 400×, 600×, 600×, 400×, 600×
Habitat: filamentous green algae (mostly Mougeotia and Spirogyra) and some organic debris collected from a freshwater pond.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 400× is 150 µm.
Fotos / Sonidos
Observ.
someplantDescripción
Magnification of photos: 100×, 200×, 200×, 200×, 400×, 400×
Habitat: filamentous green algae (mostly Mougeotia and Spirogyra) and some organic debris collected from a freshwater pond.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 100× is 600 µm.
Fotos / Sonidos
Observ.
crseaquistDescripción
Gathered dry leaves on 2024-02-23 and stored in water.
Fotos / Sonidos
Observ.
crseaquistDescripción
Gathered dry leaves on 2024-02-23 and stored in water.
Fotos / Sonidos
Qué
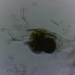
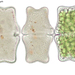
Algas Carofitas (Filo Charophyta)Observ.
swimjamieDescripción
In sample from margin of turlough (winter lake)
Qué
Algas Carofitas (Filo Charophyta)Observ.
swimjamieDescripción
In sample from margin of turlough (winter lake)